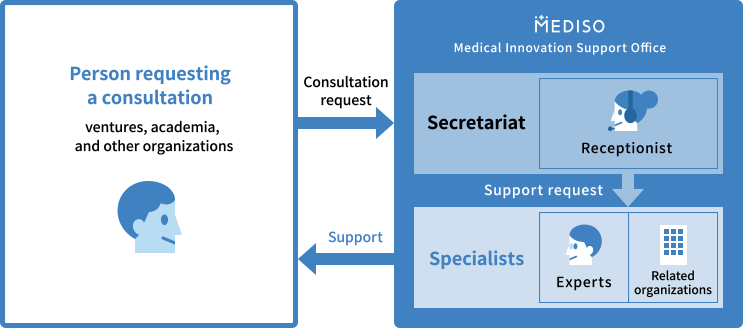
Specialists Introduction
Medical Innovation Support Office support consultants in cooperation with experts (specialists) in each field of R&D, pharmaceutical affairs and insurance, intellectual property management, management and financial accounting, marketing, legal affairs, and international development, as well as related organizations, including the Ministry of Health, Labour and Welfare.
What is Specialists
Experts in each field of R&D, pharmaceutical affairs and insurance, intellectual property management, management and financial accounting, marketing, legal affairs, and international development.
Depending on the specifics of your request, we offer support in cooperation with appropriate specialists and related organizations, including the Ministry of Health, Labour and Welfare.
Introduction of Support Providers
We will introduce registered support providers
(updated as necessary).
Search the area
Search the area
Tetsuomi Takano
t2T Healthcare Inc. CEO/Drug R&D Expert Editor-in-Chief
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Management
Strategy - Other
Tetsuomi Takano possesses over 33 years of clinical development experience spanning multiple therapeutic areas. He has accumulated extensive expertise in development strategy, project management, clinical development, regulatory intelligence, and regulatory affairs across Asian countries and regions including Japan and China. He previously held key positions in the clinical development departments at Astellas Pharma, overseeing processes from IND applications for first-in-human studies to NDA approvals in Asian countries and regions. A graduate of Tokyo University of Science's Faculty of Pharmaceutical Sciences and a RPh, his career began in 1986 at Astellas, where he worked for 31 years till 2017. After working as Senior Strategy Director, Strategy & Planning at Covance (currently Fortrea) from 2017 to 2023, he founded t2T Healthcare Inc. in October 2023. The company is dedicated to providing clients with efficient and distinctive strategic solutions to facilitate operational implementation, clinical development, and IND/NDA programs across the Asia-Pacific region. Additionally, he serves as a member of the PMDA China Expert Committee since 2020 and co-founded the web journal “Drug R&D Expert” in 2021, where he holds the positions of Editor-in-Chief and primary author.

Masato Kishida
Main specialty areas
- Medicines
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Management
Strategy - Other
My global achivements are categorized in two main types as follows: 1. Succesfully cordinating and facilitating several business globalization projects of some orphan products for CNS disorders and inherited metabolic disorders as a project leader, and 2. Outstanding success in the legal entities' establishment of three key area, e.g., a global clinical development head office in EU, two clinical and RA offices in the US & Brazil and an ASEAN region business head office in Singapore. Global business expansion is composed of two main phase. First one is to elaborate specific business plan from global business policy to clinical development strategy to draw the master of global business big picture. Next one is to implement the fixed road map and steamlined action plans on the timeline basis under the frequent risk assessment and business environment analysis.

Taku Seriu
Seriu Medical Consulting Ltd. CEO
APCER Life Sciences. Senior Advisor to the Board
The Institute of Drug Development Career Promotion. Representative Director
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Business
planning - Management
Strategy - Other
A physician and Doctor of Medical Science, he began his career as an internist before leading clinical leukemia research at the Universities of Ulm and Heidelberg in Germany. After returning to Japan, he took on leadership roles including Head of Clinical Development at Nihon Schering, Executive Officer at Bristol-Myers Squibb, and Executive Vice President and Board Member at Otsuka Pharmaceutical, where he oversaw drug development, medical affairs, regulatory affairs, pharmacovigilance, and quality assurance.
He has led the development, approval, and post-marketing activities of more than 50 therapeutics and diagnostics across oncology, neuroscience, cardiovascular and renal diseases, infectious diseases, and rare diseases, contributing to global launches and appropriate-use initiatives.
He currently serves as President of Seriu Medical Consulting and Senior Advisor at APCER Life Sciences, supporting R&D strategy, safety and risk management, organizational development, and new business planning. He also advises startups on development strategy, TPP creation, and fundraising pitch preparation, and has a strong track record in post-acquisition organizational integration, aligning cultures, talent, and processes.
He teaches pharmaceutical medicine at several universities and is an editor and contributing author of Introduction to Pharmaceutical Medicine, the first textbook of its kind published in Japan. He continues to practice clinical medicine and remains committed to supporting those driving pharmaceutical innovation in Japan, guided by the principle: “Learn Together, Grow Together.”

Masanori Sato
ReachMed Partners LLC President
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy - Other
Masanori has extensive experience in pharmaceutical R&D, alliance management, and business development. Their career evolved into leadership roles in global product launches, strategic licensing, and early-stage clinical development, particularly in antiviral therapeutics at a R&D focused pharmaceutical division of a leading Japanese conglomerate. Later, as head of the business development department, they managed global licensing agreements and partnerships with major pharmaceutical companies in the U.S. and Europe for multiple in-house pipeline assets. Since 2016, they have held leadership roles in alliance management and business development at leading U.S. biotech and European pharmaceutical companies, focusing on partnerships with Japanese firms, contract negotiations, and search & evaluation of external technologies/assets across Japan and the Asia-Pacific region. From 2021, they joined a top Japanese pharmaceutical company, where they led open innovation initiatives, incubation of internal and external seed assets, and support for venture investments. During this time, they also served as a board member for a portfolio biotech companies. In March 2025, they founded their own company, leveraging their broad expertise and network to support Japanese biotech startups and assist international startups into Japan. They hold national license for Small and Medium Enterprise Management Consultant, among others.

Hitoshi Fujimaki
TMI Associates Attorney-at-law (admitted in Japan and New York)
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Other
Joined TMI Associates in 2016. Completed an LL.M. in National and Global Health Law at Georgetown University Law Center in 2023, earning the Food & Drug Law Certificate. Admitted to the New York State Bar in 2024. The practice focuses on pharmaceutical, medical device, and healthcare regulatory matters in both Japan and the United States, with extensive experience supporting R&D and commercial operations of Japanese companies domestically and abroad. Work includes licensing and collaboration agreements with overseas partners, joint research and development arrangements, CRO agreements, GCP compliance during clinical trials, promotional review, regulatory assessments (including medical-device classification), authority inspections, and broad advisories on pharmaceutical and medical-device regulations. Also advises on U.S. healthcare compliance issues, including the Anti-Kickback Statute, the Sunshine Act, and related federal and state requirements. Author of Introduction to U.S. FDA Drug and Medical Device Law and Regulation (Shojihomu, September 2024).

Satoshi Ogawa
TMI Associates Partner (Attorney)
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Fundraising
- Management
Strategy - Intellectual
property
management - Other
Satoshi Ogawa, Ph.D., is a partner at TMI Associates specializing in life sciences and healthcare. After earning his Ph.D. in Life Sciences from Kyoto University, he joined TMI Associates as an attorney. Following a three-year secondment at a law firm in New Delhi, India, he has been based at TMI’s Kyoto Office since 2019. Dr. Ogawa advises both Japanese and international clients on cutting-edge technologies, including regenerative medicine, digital health, and AI-driven healthcare solutions. His work covers startup support, patent licensing, industry–academia collaboration, regulatory compliance, and intellectual property disputes. He works extensively with universities, startups, pharmaceutical and medical device companies, venture capital firms, and public institutions. He also serves as an advisor to BioCommunity Kansai (BiocK) and HVC KYOTO, and contributes to conflict-of-interest (COI) committees and institutional review boards (IRBs). Dr. Ogawa is passionate about advancing innovation and helping clients navigate complex legal and regulatory challenges in the life science ecosystem. His publications include “Startup Intellectual Property and Legal Guidebook – Key Points for Early-Stage Bio and Life Science Ventures” (METI-Kansai) and “Healthcare Business Legal Consultation Handbook” (Chuokeizai-sha).

Rou Irisawa
IRISAWA Consulting LLC
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Other
Worked at Chugai Pharmaceutical Co., Ltd. for 27 years, primarily in antibody drug research and development, CMC regulatory affairs, and quality assurance, from preclinical trials stage to BLA stage. Involved in the BLA of Japan, US and EU approval for the CTD-Q of Actemra, Japan's original first antibody drug. And also involved in Japan BLA for several biologics Genentech original. Since 2017, involved in the development of many new modalities, including three SAKIGAKE designated cell products, and participated in 21 times PMDA consultations responsible for CMC regulatory and QA for cell products and gene therapy products. In 2023, became independent as a CMC regulatory consultant. Author of ""CMC Pharmaceutical Design - CMC Pharmaceutical Strategy in the Era of New Modality Pharmaceuticals"" (Yakuji Nipposha, 2022), and is the overall supervisor of the Japanese translation of A-CELL (CMC Development Guidance for CAR-T). Fellow at Tama University's Medical Care Solutions Research Institute, and a top performer in the regular course of Pharmaceutical Evaluation Science at the University of Tokyo Graduate School of Pharmaceutical Sciences in 2024. Certified in generative AI skills (November 2025). Currently involved in various projects, including serving as CMC lecturer at seminars on how to proceed with the development of CMC for antibody drugs, cell products, gene therapy products, etc for small company to semi-major company.

Tomoko Maeda-Chubachi
TMC Clinical Development Consulting, Inc, President; The Institute of Drug Development Career Promotion, Founding Director; Japan Alliance of Medical School Startups, Director
Main specialty areas
- Medicines
Specialized support fields
- Laws and
regulations - Business
planning - Management
Strategy - Other
Dr. Maeda-Chubachi is an advisor and consultant for pharmaceutical and biotech companies and was the Chief Medical Officer at Pelthos Therapeutics (previously Novan), leading First-in-Human to launch preparation. Tomoko is known for her expertise in drug development with over 20 years industry experience. She has led clinical and medical affairs in large pharma as well as a small biotech. She led global drug development teams, resulting in 8 approvals in multiple regions, including Xalatan, Xeljanz, Taltz, Vtama, and Zelsuvmi. Tomoko has broad knowledge of drug development, of which process is highly regulated and need thorough risk assessment, management, and control. As a Chief Medical Officer, she worked closely with the CEO and board about the strategy and governance.

Kumi Sakurai
早稲田大学大学院理工学研究科で生体工学を専攻後、慶應義塾大学病院で10年以上にわたり血液研究に従事。その後、外資系医療機器メーカーであるベックマンコールター(2005年~)、日本メドトロニック(2007年~)、セントジュードメディカル(現アボットメディカル、2015年~)のマーケティング部門にて、医療機器の市場導入や事業戦略を担当。2019年、医療機器・ヘルスケア機器の開発支援を専門とするインキュベーター、プレモパートナー株式会社を共同創業。研究者・スタートアップ・大企業を対象に、事業戦略策定から薬事・保険償還・上市までを一気通貫で支援し、国内外のネットワークを活かして医療機器の事業化を推進している。
文部科学省 科学技術・学術審議会専門委員、東京都AMDAPカタライザー
早稲田大学大学院修了(工学修士)。金沢工業大学大学院修了(MBA)
Main specialty areas
- Medical devices
Specialized support fields
- Marketing
- Business
planning - Management
Strategy
After earning a master’s degree in Biomedical Engineering from Waseda University, Kumi Sakurai engaged in over ten years of hematology research at Keio University Hospital. She then transitioned to the medical device industry, joining Beckman Coulter in 2005, Medtronic Japan in 2007, and St. Jude Medical (now Abbott Medical) in 2015, where she worked in marketing and business strategy for innovative medical technologies. In 2019, she co-founded Premo Partners Inc., an incubator specializing in the development and commercialization of medical and healthcare devices. Premo Partners supports researchers, startups, and large corporations throughout the entire innovation process—from business strategy formulation, market analysis, and regulatory and reimbursement planning to commercialization. Leveraging extensive domestic and international networks, the company provides end-to-end support to accelerate the delivery of essential medical technologies to patients and healthcare professionals. Through its comprehensive expertise in regulatory pathways, evidence generation, and global market access, Premo Partners plays a vital role in fostering Japan’s MedTech ecosystem and bridging it with the global healthcare innovation community.

Saho Murakami
VISIONEO LLC CEO
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Business
planning - Fundraising
- Management
Strategy
After gaining clinical experience as an ophthalmologist, I served at the Pharmaceuticals and Medical Devices Agency (PMDA), where I was responsible for reviewing and providing consultations on pharmaceuticals, medical devices, and regenerative medical products in the fields of CNS, sensory organs, and anesthesiology. My work included designation reviews for Sakigake (pioneering) and orphan drugs, as well as post-marketing safety activities. Currently, I support business and clinical development across the healthcare and life sciences sectors, offering strategic and R&D advisory services to major corporations, startups, and venture capital firms.

Tomohiko Goto
TAIYO Pharma Co.,Ltd CMC Solutions Dept. Manager
Main specialty areas
- Medicines
Specialized support fields
- Other
Having extensive experience and achievements in formulation development, new drug application processes, investigational drug formulation development, support for overseas clinical trials, plant construction and GMP acquisition through long years at a pharmaceutical company, and more recently, engaging in CMC activities for investigational drug development and clinical trial advancement at a medical bio-venture company within Shonan i-Park(Kanagawa,Japan), I hope to contribute as a part-time CMC supporter for MEDISO. I am passionate about supporting the swift delivery of unmet need pharmaceuticals developed by domestic and international medical pharmaceutical venture companies to patients. Among many support areas, if I can contribute my specialized and niche knowledge in the CMC field (particularly in formulation development) to address challenges in cutting-edge bio-venture research worldwide through the MEDISO project, and support quicker entry into clinical trials, it would be most gratifying.

Tetsuo Miura
Specially Appointed Researcher, Center for Clinical Sciences, National Center for Global Health and Medicine
Main specialty areas
- Medicines
- Medical devices
Specialized support fields
- Marketing
- Business
planning - Management
Strategy - Other
I worked as a product and business development manager primarily in the fields of diagnostic equipment-reagent systems and medical devices at major foreign pharmaceutical and domestic medical device companies, and also participated in global product development teams at the US and French headquarters. I have medical science knowledge in the fields of infectious diseases, immunology, cardiovascular diseases, metabolism, reproductive medicine, and laboratory medicine, as well as information on the medical field. After mandatory retirement, I served as a senior researcher at the National Center for Global Health and Medicine, responsible for developing and implementing international collaborative clinical research and clinical trials for pharmaceuticals and medical devices with clinical trial networks in Southeast Asian countries. Currently, as part of an industrial-medical collaboration project, I also provide guidance and support to companies in product development with an eye toward exit strategies such as obtaining pharmaceutical or WHO PQ approval, and being included in guidelines in cooperation with related medical sectors and societies.

Aiko Kato Sullenberger
RykoTECH President
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy
Aiko Kato Sullenberger, CEO of RykoTECH, a significant contributor within the Boston startupecosystem for several years, brings a wealth of experience in various roles, spanning investor,startup, and accelerator. She provides specialized advisory services comprehensive strategicconsultation and facilitation of market entry into the US across diverse sectors, including drugdiscovery, medical devices, digital health, digital therapeutics and biotech. Aiko can help • Fundraising • Strategic Partnership Development • Create Deal Flow (Connecting with Potential Investors) • Identifying Partners and Advisors • Assistance in Facilitating Clinical Trials • Corporate Strategy: Investment Thesis, Portfolio Analysis • Market / Trend Research & Analysis • Go-to-Market Strategy • Academic Business Liaison: Interdisciplinary Project Lead • Cross-Functional Team Building

Tomoyo Mori
Fast Track Initiative, Inc. Capitalist
Main specialty areas
- Medical devices
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy - Other
Tomoyo obtained her medical license and began her career by engaging in training and clinical practice in medically underserved areas. She then joined the Department of Cardiovascular Medicine at Tokyo Women's Medical University Hospital, where she treated patients with heart failure, heart transplants, and myocardial infarctions. At the university hospital, she also participated in clinical trials for medical device startups. In parallel with her clinical work, she was involved in domestic and international business development for medical device startups and led a team while participating in U.S.-based MedTech accelerators (MedTech Innovator, Fogarty Innovation). She holds an MBA from the University of Michigan's Ross School of Business and is a board-certified internist by the Japanese Medical Specialty Board as well as a certified occupational physician by the Japan Medical Association. Currently, she is part of Fast Track Initiative, where she focuses on investment, company creation, and business development for medical device startups.

Hirohumi Shido
Main specialty areas
- Medical devices
Specialized support fields
- Other
I am a UI/UX design expert and specialize in driving product development. As a product manager at a medical startup, he was in charge of renewal and new planning for multiple SaMD products. My experience ranges from planning to obtaining regulatory approval, and I have won a Good Design Award for a healthcare product. These experiences have made me realize the importance of experience design and usability in SaMD products with a diverse mix of stakeholders.

Kazuhiro Terashima
CaTe inc. CEO
Fujita Health University, Department of Cardiology, Assistant Professor
Main specialty areas
- Medical devices
Specialized support fields
- Business
planning - Fundraising
- Management
Strategy - Other
Cardiologist, Fellow of Cardiovascular Intervention and Therapeutics, Registered Cardiac Rehabilitation Instructor, Certified Occupational Health Physician and Clinical Education Instructor. He graduated from Nagoya University School of Medicine in 2011. After working at the Japanese Red Cross Aichi Medical Center, Nagoya Daiichi Hospital, Sakakibara Memorial Hospital and other institutions, he joined Fujita Health University in 2023 as Assistant Professor of Cardiology and CCU Instructor. In 2020, he founded CaTe Inc. to research and develop programmed medical devices for cardiac rehabilitation, and to conduct clinical research and company-led clinical trials of such devices. He is currently the principal investigator for basic research on the Exercise Load Optimization function, a promising technology seed. In 2023 he closed Series A funding with JAFCO etc. as lead investor. While continuing his clinical work, he serves as CEO of the company and advises and supports other clinicians in the social implementation of clinical technology seeds.

Miyuki Nagao
Globizz Japan Corporation Tokyo Office Director
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Management
Strategy
After working at U.S. headquarters as an assistant for FDA registrations, FDA filings, and regulatory investigations for medical devices, pharmaceuticals, and food products, she moved to GLOBITS' Tokyo office to work as a project coordinator. While proposing and coordinating projects related to Japanese companies' entry into the U.S. market, she also provides support for attending and responding to FDA inspections in various fields, advises on FDA registration, conducts brief investigations, and teaches seminars.

Minori Kosuge
Senior Manager, Regulatory Affairs and Quality Assurance
Rokken, Inc.
Main specialty areas
- Medical devices
Specialized support fields
- Laws and
regulations - Other
- Experience of working for 15 years at a Japanese medical device company: Technical writing, regulatory affairs (US 510k submissions, translation for QSR inspection), R&D of patient monitor and its related programs.
- Experience of working at Japanese SaMD startup companies: experience of negotiation with PMDA and consultation for regulatory affairs/quality assurance.
- Currently working as a regulatory affairs/quality assurance consultant and an in-house regulatory affairs/quality assurance specialist.
Support available for class II active medical devices and SaMD, for Japan and US.

Osamu Tabuchi
JMDC Inc. Quality Assurance Office manager
Main specialty areas
- Medicines
- Medical devices
Specialized support fields
- Laws and
regulations
I am a dedicated professional with extensive experience in safety management and post-marketing surveillance (PMS) in the pharmaceutical industry. After joining SANWA KAGAKU KENKYUSHO CO., LTD., I focused on risk management for diabetes medications and treatments for dialysis complications. My work included PMS literature development and cardiovascular event evaluations, contributing significantly to drug development.
At CureApp, Inc, a company specializing in software as medical devices, I served as the Safety Management Officer. I successfully established the safety management system and operational processes for Japan's first therapeutic app. My involvement in risk management for various therapeutic applications, including those for hypertension, allowed me to engage deeply with the core aspects of product development.
Currently, I am the Head of Quality Assurance at JMDC, Inc, where I oversee GxP compliance and reliability assurance. I am actively involved in building a new PMS framework that combines e-source data with additional collected data, as well as managing certification acquisition and maintenance.

Mineki Takechi
Cardio Intelligence Inc. Chief Development Officer
Director
Main specialty areas
- Medical devices
Specialized support fields
- Business
planning - Management
Strategy - Other
After working at Fujitsu Ltd. on the development of products and services such as image processing system and cloud information service for home healthcare, as well as research and development in information retrieval and AI, and new business development, I engaged in the construction of a pulmonary hypertension patient registry at the International University of Health and Welfare Mita Hospital. Since 2020, I have been serving as CDO at Cardio Intelligence Inc., responsible for the development of software medical devices for arrhythmia analysis and regulatory affairs. Since 2021, I have also been serving as COO, overseeing overall business planning and execution.

Kazufumi Nakamura
Mitsui & Co. Global Investment, Inc. Investment Director
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Business
planning - Fundraising
- Management
Strategy - Other
Kazufumi Nakamura is an Investment Director focused on the investment in biotech in US and China. By leveraging his experiences and networks in Pharma, Biotech and Academia, he connects companies to those experts to expand business and gain scientific insight to help the companies succeed with their important missions.
Kazufumi has over 20 years of healthcare and life sciences experience, spanning business development, investment, entrepreneurship and R&D. Prior to joining MGI, he co-founded a biotech to accelerate innovations through academic institutions in Japan. Previously, he engaged in business development and conducted scientific evaluation, and license-in and license-out of pharmaceutical products at Santen Pharmaceuticals. In addition, he was responsible for creating relationships with the leading academic institutions and innovators, gaining Santen early access to innovation. Prior to Santen, he held a number of clinical development roles at Janssen Pharmaceuticals, including the development of Alzheimer’s disease and Rheumatoid arthritis programs.

Akiko Yamamoto
Nissan Chemical Corporation
Environment, Safety and Quality Assurance Department
Quality Assurance Group
Senior Advisor (contract employee)
Main specialty areas
- Medicines
Specialized support fields
- Laws and
regulations - Management
Strategy - Other
Graduated from the Department of Pharmacy at Kyoritsu Collage of Pharmacy (currently Keio University) and received a Ph.D. from Gifu Pharmaceutical University. Engaged in work related to each process of pharmaceutical life cycle (research and development, sales promotion, licensing/alliance, quality assurance and Good Vigilance Practice(GVP)) at Nissan Chemical Corporation. Especially, as the corporate quality assurance department leader, managed the company's pharmaceutical quality system, and maintained and improved appropriate quality assurance systems for API manufacturing sites, manufacturing contractors, and contract testing facilities. Specifically, provided support during FDA inspections and global pharma quality audits, and advised on improvements and response policies for issues raised. Also has experience in implementing regulations and change processes when adding starting material, intermediate and API manufacturing sites, and testing facilities. In addition, has reviewed documents such as license and quality assurance-related contract contents, change applications and master files to be submitted to authorities.

keiji Asada
Amenichi Consulting, LLC, CEO
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Fundraising
- Management
Strategy - Other
Graduated from Auburn University with a degree in Chemical Engineering. Currently, serving as the CEO of Amenichi Consulting, LLC, a consulting firm specializing in regulatory compliance (FDA) and marketing support for companies entering the U.S. market, particularly in the fields of pharmaceuticals, medical devices, and regenerative medicine products. With extensive industry experience and expertise, he assists clients in developing business plans, securing funding, and establishing operational structures. Additionally, he provides comprehensive support, from the formulation to the execution of business strategies, to help clients enhance their competitiveness. Furthermore, he leverages his network to match clients with the most suitable business partners. With a global perspective, he offers customized services tailored to the needs of each client, aiming for sustained growth and success. Amenichi was established in 2018 and has supported over 50 companies since its inception.

Ruka Takeuchi
Discovery Biotech Consulting Representative
PARKS Commercialization Producer
Main specialty areas
- Medical devices
Specialized support fields
- Marketing
- Business
planning - Management
Strategy - Other
I have been engaged in marketing and business development for more than 30 years in the life science market, mainly in the medical and pharmaceutical fields, both in Japan and overseas. Currently, I have established my own consulting firm and provide business consulting services to foreign and domestic companies from start-ups to small and medium-sized enterprises. I would be happy to be of service to you by drawing on my past business experience as well as my daily experience in providing hands-on support to various companies and projects.

Saaya Hirai
Globizz Japan Corporation Osaka Office Director
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Management
Strategy
After studying abroad in New Zealand during junior high school, she became interested in doing business with foreign countries, and after graduating from high school, she entered a university in Taiwan in English and Mandarin Chinese. At university, she majored in international business administration. During her studies, she experienced corporate internships in the U.S. and Taiwan, gaining insight into the business world from her student days. Currently, as a project controller at headquarters, she is in charge of overall project management and strategic planning for companies entering the U.S. market as a project coordinator.

Teppei Konishi
Biomy Inc. CEO
Main specialty areas
- Medical devices
Specialized support fields
- Laws and
regulations - Business
planning - Fundraising
- Management
Strategy - Other
He completed his graduate studies at the Graduate School of Engineering Science, Osaka University. At NTT DOCOMO, he specialized in big data analysis and AI-driven video analysis research, contributing to new business development. After leaving NTT DOCOMO, he served as CTO at an IT venture before founding Biomy Co., Ltd. There, he led the development of a personalized medical platform utilizing AI technology, collaborating with domestic and international pharmaceutical companies and research institutions. He also secured a Class II medical device manufacturing and sales license.

Hikaru Saito
Saisei Ventures LLC Partner
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
As a partner of Saisei Ventures, a newly established global venture capital firm that invests in advanced biotech companies in cell and gene therapy, Dr. Saito is responsible for Japan operations including company creation, integrating Japanese science and technology with the Western ecosystem. Prior to joining Saisei, he started career as a research scientist at Astellas Pharma Inc. and then as a Senior Manager of Business Development and as a Senior Investment Manager at Astellas Venture Management, a CVC arm based in Silicon Valley, USA, where he was a lead role for deal process, due diligence and transactions for venture investments in RNA therapeutics, cell and gene therapy. He was providing business support to portfolio companies based on the expertise, experience, and global network. He also led the development of strategic partnerships with venture capital funds, accelerators, and institutional investors based in the U.S. and Europe. Prior to joining Astellas, he was a Visiting Fellow at the Institute of Medical Science, University of Tokyo, and a Research Fellow of the Japan Society for the Promotion of Science. He received his B.S. in Biotechnology and M.S. and Ph.D. in Biomolecular Molecularl Engineering from Tokyo Institute of Technology.

Hiroyuki Kajiyama
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning
Graduated from Kyoto University Faculty of Pharmaceutical Sciences. Worked at Tanabe Mitsubishi Pharma Corporation in pharmaceutical research and development, primarily handling clinical development tasks. Since 2016, employed at the Pharmaceuticals and Medical Devices Agency (PMDA), Review Management Department, as a Technical Expert in Regulatory Science Strategy Consultation. Engaged primarily in providing consultation services on pharmaceuticals, medical devices, regenerative medicine products, focusing on academia and venture companies.

Akiyo Inoko Hewett
Attorney, Smith, Gambrell & Russell, LLP
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
Akiyo Inoko Hewett is a bilingual and double-licensed attorney based in Atlanta, Georgia, United States. She graduated from Tokyo University of Foreign Studies and earned her J.D. at Keio Law School. After passing the Japanese bar exam and completing her legal training in Kushiro, Hokkaido, Akiyo relocated to the United States. She earned an LL.M. from Emory University School of Law in Atlanta, Georgia. Currently, Akiyo serves as an attorney at Smith, Gambrell & Russell, LLP, where she provides legal services to Japanese companies doing business in the United States and supports clients across a broad range of corporate matters. Her practice areas include international and domestic commercial transactions, regulartory compliance, healthcare law, and immigration law.

Hirokazu Yamaguchi
Main specialty areas
- Medical devices
Specialized support fields
- Laws and
regulations - Intellectual
property
management

Yuki Yamakage
Representative Director, Takashi Shimada Office Co., Ltd.
Managing Partner, BD Consulting Ltd.
Main specialty areas
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Other
I have experience working for a global medical device company in insurance strategy development and insurance applications for products in a wide range of medical specialties and disease areas, as well as market access work (relevant academic societies, patient groups, government agencies and industry associations). I have now set up my own private practice and provide support to medical device and pharmaceutical companies and new entrants in terms of healthcare and care systems and insurance strategies. I have experience in the insurance application category (C1/C2 applications) for innovative medical devices. I also have a lot of experience in training employees and conducting seminars, most recently on SaMD insurance applications. My motto is to provide consulting services that address the concerns of my clients after explaining information on insurance applications for medical devices in a way that is easy to understand for beginners.

Yoshimi Naruo
Orizuru Therapeuics, Inc. Associate Director, IP&Legal Ph.D., Patent attorney
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Intellectual
property
management
Education: Ph.D. in Bioinformatics/Systems Biology, Tokyo Medical and Dental University. Conducted research in systems biology at RIKEN for 3 years (student internship). Experience: 5 years of experience in drug regulatory affairs at an originator pharmaceutical company, followed by 2 years as an in-house IP specialist handling invalidation and infringement matters at a generic pharmaceutical company. Patent prosecution primarily in the fields of pharmaceuticals, biotechnology, and food at an IP law firm. Fulfilling a long-held aspiration to join a startup, she took on the role of Vice President of Legal IP & Strategy at Splink, Inc., where she contributed to winning the Grand Prix at the 3rd IP BASE AWARD hosted by the Japan Patent Office and the 2022 WIPO Global Awards hosted by the World Intellectual Property Organization (WIPO). Currently, she is responsible for intellectual property and legal affairs at Orizuru Therapeutics, Inc., a company aiming to commercialize regenerative medicine derived from iPS cells. She leads the formulation of IP strategies and the development of an IP portfolio. Her strength lies in creating IP strategies with a focus on pharmaceutical regulatory affairs, and she strives to provide practical advice in the face of limited resources and uncertainties surrounding technology and commercialization.

Hiroki Yamada
Medii, Inc. CEO, M.D.,Ph.D.
Main specialty areas
- Medicines
Specialized support fields
- Business
planning - Fundraising
- Management
Strategy - Other

Tomohisa Hayasaki
GVA LPC Attorney-at-Law / Partner
The Leader of The Team ""Medical / Healthcare / Cosmetic Industry""
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Fundraising
- Intellectual
property
management - Other
After registering as a lawyer, I have been handling disputes such as corporate and labor disputes, as well as providing legal support for the designing lawful business models of new businesses and for funding. In addition, I have been providing legal support for businesses in the field of medical and beauty based on my knowledge of laws relating to medicals including the Japanese Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices and the Japanese Medical Care Act. In particular, I have been providing extensive support for numerous businesses operating in new field such as Software as Medical Device (SaMD) and Non-Software as a Medical Device (Non-SaMD), telemedicine/home call medical services, and also for companies utilizing medical information by employing new technologies such as AI. I am also addressing legal issues faced by companies using advance technologies. Furthermore, I have expertise in the latest marketing trend and offer advice on marketing strategies based on the Japanese Act against Unjustifiable Premiums and Misleading Representations, advertising regulations in Japan such as medical advertising regulations, and medical and pharmaceutical devices regulations.

Tomokazu Ichikawa
Director, TM Research and Investments Pte. Ltd.
Main specialty areas
- Medical devices
Specialized support fields
- Laws and
regulations - Business
planning - Fundraising
Leveraged cross-functional expertise in business strategy and legal to work on strategy development, M&A, and investments in the medical device sector. Practiced law at Nagashima, Ohno and Tsunematsu, handling M&A and other corporate matters. Worked for McKinsey & Company and Hoya Corporation. Completed several acquisitions and investments in the US and Europe in the medical device business. Experienced managing a healthcare service operator in India. Currently, helping AI Medical Service, an artificial intelligence software startup for endoscope and CUC, a healthcare service provision related company. Spent more than 10 years in Singapore and familiar with business in Southeast Asian countries. Graduated from Waseda University, London Business School's Masters in Finance, and Cambridge's MBA. Attorney at law admitted in Japan.

Tsutomu Hoshiba
Kievit Scientific LLC President
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Other
For over 30 years, I've established and managed Japanese life science companies in the US and Europe. In 2021, I founded Kievit Scientific in Philadelphia, utilizing my Western-acquired experience, up-to-date information, and network. I assist Japanese companies in the pharmaceutical, medical device, and chemical sectors in their international expansion, from strategic planning to product development and market entry. Harnessing my understanding of different cultures and wide-ranging scientific knowledge, I provide solutions to challenges faced by Japanese academia and startups in their global ventures, with the aim of supporting as many Japanese companies as possible to succeed overseas. Furthermore, through these endeavors, I promote the widespread adoption of innovative medical technologies, striving to realize a society where the benefits are extended to many.

Mirai Miyahara
President of i Access Consulting Inc.
Main specialty areas
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Fundraising
- Management
Strategy
Miyahara holds a Master's degree in Business Administration from the Graduate School of Economics at Tohoku University. She possesses 17 years of professional experience in Regulatory Affairs, QMS, business planning, and overseas marketing at a leading medical device company and Notified bodies in Japan. With a specific focus on multi-country regulatory compliance and extensive experience in overseas medical device markets, Miyahara has demonstrated proficiency in navigating the regulatory landscapes of diverse markets, including Japan, China, India, East Southern Asia, EU and the U.S. She has a proven track record of achieving success in supporting medical companies with regulatory compliance, facilitating international expansion, and establishing strategic partnerships with local entities. Miyahara is also an adviser for JETRO medical devices international expansion program.

Satoshi Morimoto
Morimoto Phrma Partrnering, Representative
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Business
planning - Management
Strategy - Other
Representative of Morimoto Pharma Partnering. Master of Science in Applied Biochemistry, Graduate School of Waseda University. I stared my carrier at the former The Green Cross Corporation (now Mitsubishi Tanabe Pharma Corporation), experienced in pharmaceutical R&D, product strategy planning, and business development. During that time, I was dispatched as the liaison to Germany for four years. My last position was Director of Business Development. Then, I joined to CMIC Holdings, where I led the business development of rare disease drugs as Director of Business Development and Head of IPD Company. From 2016, I spent six years at the Mitsubishi Chemical Group's Life Science Institute(LSII) as head of the Regenerative Medicine Division, where I was involved in the early stages of cell product development, initiating clinical trials, and leading the construction and operation of cell processing facilities. After retiring from LSII in 2021, I started own business as a consultant. I would like to provide practical advice based on my experience in the development and commercialization of rare disease drugs and regenerative medicine products. In addition, I would like to provide practical advice on business development from the perspective of having been introduced to various projects from venture companies over 30 years.

Kazuhiro Ikumi
MEDICOLAB Co., Ltd.
Main specialty areas
- Medical devices
Specialized support fields
- Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management - Other
Board-certified neurologist, CEO at MEDICOLAB, Co., Ltd. My initial job was a medical doctor at a tertiary care emergency hospital. I have 14 years of clinical experience in Parkinson's disease, dementia and other neurodegenerative diseases. MEDICOLAB, Co., Ltd. receives PR and development support from Fujitsu, NVIDIA, Amazon, and Microsoft. I am involved in business development in the CNS area and support researchers for their idea and trial products or services for commercialization.

Yutaka Kono
Guest professor
Drug Discovery Science Division,Institute for Open and Transdisciplinary Research Initiatives, Osaka University
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Business
planning - Fundraising
- Management
Strategy - Other

Masaho Ishino
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Management
Strategy - Intellectual
property
management - Other
After engaging in medical research for 25 years, I became a patent attorney (JAPAN). Over the next 20 years, I have supported medical researchers in creating intellectual property and planning research strategies, as well as engaged in contract practice and technology transfer of university IP. Intellectual property strategies for regenerative medicine and other new modalities require not only a high level of expertise in both cutting-edge medical research and intellectual property rights, but also comprehensive knowledge covering clinical development and regulations. Utilizing my experience of contributing to the development research and practical application of university-originated seeds as a representative of an institution responsible for the AMED's Project of Translational and Clinical Research Core Centers and an organizer of nationwide academia intellectual property networks, I aim to contribute to raising the potential of intellectual property of academia/venture.

Katsuhiro Fukunishi
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Other

Kunishige Masui
Managing Partner
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Fundraising
- Intellectual
property
management
Graduated from Kyoto University Faculty of Law, University of Tokyo Law School, and University of California, Irvine LLM. 2014 - 2021 at Nagashima Ohno & Tsunematsu; 2020 - 2021 at Smith, Gambrell & Russell, LLP (US law firm). Established Masui & Partners in December 2021. Mr. Kunishige Masui assisted numerous startups, from angel rounds to companies nearing IPO, and has also assisted in the launch of many new businesses, including cross-border localization. He is well versed in the legal and financing issues that arise for startups and new business development, and provides support as a mentor for JETRO, Healthcare Innovation Hub, Kawasaki-NEDO Innovation Center (K-NIC), Venture Café Tokyo, and IDEC. In addition to providing easy-to-understand explanations of foreign legal systems in comparison to those in Japan, he also specializes in removing obstacles to business growth by not only providing answers to "points that clients have actually consulted with" but also by digging into "points that need attention that clients are not yet aware of.”

Akihiko Watanabe, Ph.D.
President, GlobalHumanScienceInnovation; Senior Consultant, Headland Strategy Group; Visiting Professor, Gifu Pharmaceutical University; BD Consultant, United Immunity; Executive Advisor, SOKK Japan
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy
With R&D expertise atJapanese and global pharmaceutical companies as a backbone, he has been engaged in marketing, BD, management planning and fundraising, in addition to R&D. In the Editorial Committee of the Pharmaceutical Society of Japan, he organized roundtable discussions and special issues on practical application of academia seeds. He has been thinking about how Japanese bio-ventures can succeed. Over the past five years, he has worked at bio-venture companies as CBO or VP of BD and open innovation. He is also engaged in the making and analysing stratetgies of management, business, R&D, and marketing for pharmaceutical companies and bio-ventures on commission from a US consulting firm.

Eiichi Yamaguchi
Eiichi Yamaguchi, Ph.D. President A1 Partners
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy
Ph.D from Faculty of Pharmaceutical Sciences Kyushu University. Currently engaged in management and business development consulting for pharmaceutical companies, biotech ventures, and consulting firms. Over 20 years expertise of business development at Shionogi Co., Ltd., a major pharmaceutical company as well as bioventure companies. Senior Director, Business Development & Corporate Planning Dept., Shionogi. A lot of global business experience such as the first Managing Director, Shionogi Singapore, Vice President, Shionogi Qualicaps, Inc., North Carolina, and Shionogi New York. Board of Director & President & COO, a bioventure company. The first chairman, Japan Pharma Alliance Association. The Vice Chairman, Public Private Alliance Conference associated with Infectious diseases led by Dr. Shigeru Omi. Also engaged in Director, International Committee, JPMA. Last but not least, selected as Mentor for Blockbuster Tokyo 2021

Kensuke Suzuki
Nagashima Ohno & Tsunematsu
Partner, Attorney-at-law
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
Kensuke Suzuki is a partner at Nagashima Ohno & Tsunematsu. He represents both domestic and international clients, with a special focus on cross-border transactions, with respect to pharmaceutical, medical and healthcare business, M&A and corporate transactions, fund formation and management, and financial regulations. With expert knowledge of the Japanese medical, pharmaceutical, medical device, and other various healthcare-related regulations, he advises on a wide variety of business activities in the broad healthcare sector, such as regulatory compliance, data protection, mergers and acquisitions, alliances, business startups, financing, licensing and other commercial transactions. He was admitted to the Japan Bar in 2000 and worked as visiting attorney at Kirkland & Ellis (Chicago) from 2006 to 2007. He served as a special adviser to the Ministry of Health, Labour and Welfare from 2014 to 2015. He is fluent in Japanese and English.

Hiroya Kuwahara
Department of Neurology and Neurological Science / Institute of Innovation Advancement
Tokyo Medical and Dental University
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Management
Strategy
M.D. (graduated from Tokyo Medical and Dental University), and Ph.D. (completed doctral course at Graduate School, Tokyo Medical and Dental University). Specialist and Attending Neurologist of the Japanese Society of Neurology, and Fellow and Board Certified Member of the Japanese Society of Internal Medicine. He is engaged in 1) clinical practice, research, and education in neurology, 2) research and development of fudamental technologies for drug discovery (especialy nucleic acid drugs and drug delivery system), 3) promotion of clinical research and industry-academia collaboration, at Tokyo Medical and Dental University. He has a career of a Deputy Director at Research and Development Division, Health Policy Bureau, at the Ministry of Health, Labour, and Welfare from 2018 to 2019, and was involved in establishing a nationwide system for promoting clinical research and accelerating venture companies in the healthcare field. He provides consultations on the needs of clinical practice, on collaborative research and field provision in relation to project implementation, and on clinical research and development.

Tomohito Suzuki
Tohoku University Knowledge Cast Co., Ltd.
Senior Consultant, Medical Device Development Support Division
Main specialty areas
- Medical devices
Specialized support fields
- Laws and
regulations - Business
planning - Fundraising
D. in Pharmaceutical Sciences from Kyoritsu Pharmaceutical University (now Keio University). Formerly a medical device reviewer at the Pharmaceuticals and Medical Devices Agency (PMDA). At PMDA, a member of the team in charge of the cardiovascular field and was in charge of approval review and consultation for many medical devices including new medical devices. Seconded from PMDA to the University of Tokyo Hospital as a Project Assistant Professor at the Translational Research Center, where supported the development of medical seeds in academia. Seconded from PMDA to Division of Medical Device Research of the Department of Industrial-Academic Collaboration of the Japan Agency for Medical Research and Development (AMED) as a manager in charge of overseeing projects with medical device-related budgets. He has been in his current position since 2020. Based on his experience in reviewing at PMDA and supporting R&D in academia and AMED, he supports efficient development of medical devices for many manufacturers including venture companies.

Akihiko Okuno
SK Intellectual Property Law Firm (SKIP) Managing Partner
SCM BioMedica Co., Ltd. Co-Founder
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Intellectual
property
management
Master's degree in Cell Structure Research, Department of Applied Bioengineering, Graduate School of Agriculture and Life Sciences, University of Tokyo, Japanese Patent Attorney, Information Security Administrator, and JPAA Intellectual Property Management Consultant certification. As a FoundX supporter of the University of Tokyo, He supports the intellectual property strategies of many IT and bio-related startups. He specializes in supporting the planning of intellectual property strategies in a variety of fields including biotechnology, pharmaceuticals, and medical devices. In addition, together with Dr. Yoshimasa Tanaka of Nagasaki University (a genius researcher who developed Opdivo with annual sales of over 1 trillion yen together with Dr. Tasuku Honjo, winner of the Nobel Prize in Medicine and Physiology), he established SCM Biomedica Co., Ltd. He co-founded the company and is currently trying to manage his own drug discovery startup.

Masakazu Masujima
Partner, Mori Hamada & Matsumoto
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Fundraising
- Management
Strategy - Intellectual
property
management
Masa is a guru of startup legal advisory space in Japan. Based on the experiences at Palo Alto office of Wilson Sonsini Goodrich & Rosati, his advice is West Coast style; progressive, risk-base and proactive. Through more than 2 dacades of dedication to the startup space, he has advised IPOs, M&As and major cross-border fundraising for may startups, including biotech and healthcare ones, and have also worked with entrepreneurs through a variety of touch situation, such as disputes between founders, problems with investors as well as intellectual property disputes. Masa has served as a council member of numerous Government committees, such as Regulatory Reform Promotion Council, the Regulatory Sandbox Committee, the Digital Market Competition Council and the Industrial Structure Council. He is admitted to NY bar and Japan bar, and a licensed patent attorny in Japan.

Yoshiki Kawabata
President, MedX Inc.
Main specialty areas
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
Advantage from over 35 years of multiple company & responsibility experience in medical device industry, helping foreign companies to enter into Japanese market. Key highlights in the past achievements are : with Johnson & Johnson Mdecial Company, worked with US R&D team to develop new product which reflecting Japanese patient and physicians needs - three years resided in Cincinnati, Ohio: HeartFlow Inc., which developed the first "program device" - FFRCT, enabling non-invasive detection of FFR, applied and received regulatory approval and reimbursement as a first product and company. : in Palette Lifesciences Japan, establishment of new subsidiary for start-up company which transferred the business from exclusive distributor. Started with minimum number of direct resources and having multiple partnerships with logistics, finance and regulatory affairs, revitalized the business and achieved a 149% growth over the last year of distributor.

Nao Yoshizawa
GRiT Partners Law offices/Willsame.co.ltd/Cabinet office advisor for National bioecnomy strategy/Keio university Medical school
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
After leaving the law firm Nishimura Asahi, [the individual] established the law firm GRiT Partners and assumed the role of managing partner. They also serve as the representative of Willsame Corporation and hold qualifications as a lawyer, patent attorney, CFE (Certified Fraud Examiner), Applied Information Technology Engineer, Information Processing Security Support Engineer, and IT Strategist. They have served as a committee member for the formulation of standards in electronic commerce and related information transactions at the Ministry of Economy, Trade and Industry, as an expert in the Cabinet Office's bioecnomy strategy, and as an advisor to Biock. They have been involved in the establishment of the Japan Chapter of Aging2.0 in Silicon Valley and served as a mentor for Alchemist Accelerator. A graduate of the law faculty at Hitotsubashi University, they completed intellectual property and machine learning programs at Stanford University, as well as multiple AI programs at MIT. They serve as a program committee member for the Graduate School of Pharmaceutical Sciences at the University of Tokyo and the Medical Innovation Human Resources Development Course at Tokyo Medical and Dental University. With a deep understanding of international perspectives on digital health, they provide advisory support for startups and pharmaceutical companies' DX (Digital Transformation) programs. They founded the Life Science Incubation Council and focus on launching projects and connecting them with ecosystems both domestically and internationally.

Kiyoaki Kojima
Partner
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
Kiyo advises clients in corporate formation, governance, and compliance matters; mergers and acquisitions; joint ventures; leasing and licensing matters; distribution and franchise arrangements; and a wide range of commercial transactions.An important part of Kiyo’s practice includes his work with international companies in relocating and/or establishing ventures in the U.S. He assists companies and investors with incentives and site selection; real estate acquisitions and construction agreements; corporate formation and governance; asset purchases and joint ventures; regulatory compliance; and contracts of all types to help ensure a seamless U.S. launch or transition.He also counsels clients in virtually all areas of labor and employment law, including drafting and/or interpretation of employment agreements, company policies, and handbooks; union-avoidance training; countering discrimination and harassment claims; hire, fire and discipline issues; and dispute resolution procedures. Active in the community, Kiyo is a member of the Atlanta Bar Association, a board member of the Japan America Society of Georgia, and a board member of the Japanese Chamber of Commerce of Georgia. He also serves in “advisor” or “supporter” roles in various entities in Japan, such as InnoHub, MEDISO, and IDEC Yokohama.

Tadashi Sameshima
Main specialty areas
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Management
Strategy
I have been engaged in product development and business operations of medical devices and regenerative medicine products for 37 years at Terumo Corporation. I have experienced the development of medical devices such as extracorporeal circulatory and blood transfusion devices, as well as the development to commercialization of cellular products. In addition, I am able to provide specific advice on various stages of development based on my participation in joint research with universities, collaboration among companies, discussions with government agencies, and activities of industry associations.

Tomokatsu Hongo
Keio Innovation Initiative, Inc
Corporate Officer
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Business
planning - Fundraising
- Management
Strategy
Ph.D. in Biological Chemistry from Nagoya University. At Summit Pharmaceuticals International Corporation, I was engaged in VC fund management, investment and sales support for bio-ventures, and domestic sales agency business for overseas bio-ventures. In 2016, I joined Keio Innovation Initiative (KII) as an investment manager in the medical and health field. I have been investing in drug discovery, regenerative medicine, and medical device ventures by leveraging his business experience in the pharmaceutical industry and career as a researcher, and providing a wide range of hands-on support, not only in terms of funding, but also in business development.

Takahiro Mizumoto
Ant innovations Co., Ltd
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
Kyoto University Graduate School of Medicine, Master’s Program in Intellectual Property Management.
After passing the Japanese Patent Attorney Examination, gained venture investment experience in high-tech sectors including IT and healthcare at NIFS SMBC Capital (later split into Daiwa Corporate Investment and SMBC Venture Capital).
Subsequently joined Showa Shell Sekiyu, where he led the planning and development of multiple new services spanning payment solutions, retail electricity, and mobile app businesses.
With the launch of the University of Tokyo Innovation Platform (UTokyo IPC), he joined the firm and founded 1stRound, a cross-university incubation program. He later spearheaded the establishment of an approximately JPY 25 billion open-innovation promotion fund and served as its Chief Investment Officer.
In November 2025, he founded Ant Innovations, a newly established venture capital firm and subsidiary of the private equity firm Ant Capital Partners, and assumed the role of Representative Director and Partner.

Chia-Feng Lu
Shareholder(Senior Partner), Greenberg Traurig, LLP
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
Chia-Feng Lu represents life sciences companies and industry associations in strategic regulatory and legislative worldwide to guide and develop new legislation and policy, to frame effective approaches to working with the various agencies on compliance and investigation issues. He has intensive experience in provision of the guidance to companies introducing novel technology products, such as AI, block chain, 3D-printing, cancer immunotherapy, digital health, cell / gene therapy, precision medicine-related products and services, RNAi drug, as well as SMART device. In his past experience, he has already assisted companies in translating the scientific ideas from Nobel Laureates into the successful commercial launch of at least two products. In addition, he advises investment banks, private equity firms, and venture capital groups on their evaluations of the technologies, deal structures, and the resulting business impacts, as well as regulatory uncertainty with respect to novel technology and compliance. He specializes in various issues in connection with biotech investors or life sciences companies’ research & development strategy, portfolio management, corporate strategy planning, partnerships, licensing, project finance, auction, investment, and M&A. He has also served as an adjunct faculty member of a number of leading academic institutions in the U.S. and Japan, and an advisor to numerous government agencies.

Takeshi komatani
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Management
Strategy - Intellectual
property
management - Other
Takeshi S Komatani is currently principal litigation certified patent attorney in the field of chemical/pharma and bio/life sciences at a patent law firm, and has a number of counsels, including in academia, start-ups and big pharma internationally. Takeshi received his PhD from the University of Tokyo, Japan, received his LLB and LLM (global legal practice) from Keio University, and is currently a visiting professor at Kobe University, teaching IP strategy in entrepreneurship and Doshisha University (Kyoto). He has completed CEIPI Summer School on IP (University of Strasbourg), France. He is also qualified as a pharmacist with a certificate of Kampo and natural medicines specialist qualification. He was a researcher at F Hoffmann-La Roche in Basle, Switzerland. He has completed the EU-recognised PharmaTrain course at Osaka University, and is qualified as a board-certified member of the Japanese Association of Pharmaceutical Medicine. He is a member of the AIPPI and vice chair of TRIPS SC and a member of IP-GRTK SC and Pharma SC, and a member of the editorial board of the Pharmaceutical Patent Analyst (UK). He is also a member of JPAA, APAA, IPAJ, PSA, JPA, CSAIP and AAAS. He has lectured in academia, including at Japanese and German universities and WIPO Academy, and contributed to a number of articles.

Motohiro Kobayashi
University of Yamanashi Hospital, Clinical Trial Management Office, Associate Professor
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Business
planning - Management
Strategy
With over 30 years of practical experience in pharmaceutical companies, ranging from basic research to clinical development, licensing activities, and regulatory audits. Subsequently, I served as a technical expert in the Innovation Commercialization Support & Strategic Consultation Division of the Pharmaceuticals and Medical Devices Agency (PMDA), dealing with regulatory consultation services for academia and venture companies (especially for regenerative medicine products and pharmaceuticals). Currently, I am working in the clinical research support department of a university, supporting physician-led clinical trials and clinical research. I have experienced numerous pre-meetings and face-to-face advice with PMDA. I also have experience in application support for public research and development projects (such as Seeds C, etc.) by the Japan Agency for Medical Research and Development (AMED). In particular, I can provide detailed support for the development of regenerative medicine products (including quality and non-clinical).

Jun Yamadera
Eyes, JAPAN co. ltd.
Main specialty areas
- Medical devices
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Fundraising
- Management
Strategy - Other
Jun Yamadera founded Eyes, JAPAN co. ltd. in 1995, the first IT startup from the University of Aizu, Fukushima. In the past 20 years, he has been working on various cutting edge projects such as exporting Fukushima rice via web in 1995, making world’s first virtual pottery system, making CG of historical archives of national treasure of Japanese castles, temples, traditional dances and medical motions using motion capture. He is a pioneer in Augmented Reality wearable technologies, Medical x IT and has been organizing world’s first medical security hackathon since 2012. His team won the the championship in Developers Challenge 2013 Health 2.0 in Silicon Valley. He is the Health 2.0 Fukushima Chapter Leader and a TEDxKobe 2015 Speaker. He started a project called “FUKUSHIMA Wheel” in the aftermath of the terrible disaster caused by the earthquake and nuclear accident in Fukushima, JAPAN in March 11, 2011. More than 24 years experience in Cutting Edge Technologies such as AI, Blockchain and Cyber Security. He is also advisor of Safecast and LivingAnywhere.

Masatoshi Tachibana
BPM Consulting Office Representative
Main specialty areas
- Medicines
- Medical devices
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Management
Strategy
He worked in the medical device division of a precision equipment company for over 25 years, working in a series of tasks spanning the product life cycle, including marketing (domestic and overseas market research, business development), product planning, legal and regulatory compliance, quality management system construction. He has experience managing numerous projects, including those outside the company also. His policy in conducting business is to maximize the performance of the organization by promoting overall optimization, making decisions based on a bird's-eye view, and building good relationships with all of stakeholders. Based on these knowledge and policies, He provides fact-based and risk-based support to ventures and small and medium-sized enterprises aiming for practical application in the medical field.
-e1571192029577.jpg)
Yumi Kawata ,MD, PhD
Hedgehog MedTech, Inc.
Main specialty areas
- Medical devices
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Fundraising
- Management
Strategy
She is Founder and CEO of Hedgehog MedTech, Inc, a start-up Digital Therapeutics (DTx) company. Previously, she was a manager with SoftBank Corp, where she evaluated new Healthcare & Life Science investment opportunities, managed relationships with strategic partners. Prior to SoftBank, she was a Government Relations specialist with Medley, Inc, where she built and maintained the relationships with Ministry of Health, Labour and Welfare and Academia. She launched researches and POCs, contributing to the spread of telemedicine in Japan. She also served as a technical officer for MHLW. she was involved in the management of researches on Rare and Intractable diseases and Infectious Diseases.

Yasuhisa Matsukawa
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Management
Strategy - Intellectual
property
management

Hitoshi Takuma
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Fundraising
- Management
Strategy - Intellectual
property
management

Shinichi Watanabe
WNW IP ATTORNEYS
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Intellectual
property
management
Shinichi Watanabe began his career as a patent attorney in 2003, and with the valuable experience gained at global patent law firms. His practice primarily deals with filing and prosecution of domestic and international patent applications in the pharmaceutical and biotechnology fields. Backed up by a wealth of practical experience and technical expertise, he is actively engaged in IP counselling and advises start-up pharmaceutical and biotech companies on patent filing strategies. Recently, he is interested in AI and machine learning, and now also working on support in the interdisciplinary area between life science, IT and AI.

Masashi Matsunaga
Biospire Japan Ltd. / Biospire UK Ltd.
CEO
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy
As an experienced global business development strategist, Dr Masashi Matsunaga provides business soft-landing, clinical and regulatory support to the UK and European market. He also enables to support IP management, marketing and pitch-deck brush-up in the MEDISO support package. He is based at Harwell Science & Innovation Campus, a core UK innovation hub located near Oxford.

Shunichi Takahashi
Bayer Yakuhin, Ltd.
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy
After joining Mitsui Pharmaceuticals Co., Ltd., he worked at Nihon Schering K.K., Berlex Biosciences (a US subsidiary of Schering AG). In 2007, he joined Bayer due to management integration. Over the past 15 years, he was in charge of exploring new projects and verifying concepts in drug discovery research, especially in the areas of cardiovascular systems, immunity, stem cell research, etc. Subsequently, he was in charge of the project management cardiovascular system area manager of development headquarters, the primary care manager of the medical affairs headquarters, and he has been in his current role since 2014 (Open Innovation Center director). He leads strategic partnerships and joint research with Japanese academies and venture companies. He is also verifying new pharmaceutical business models that take advantage of big data, AI, IoT, and others. In 2018, he launched the first venture incubation facility (CoLaboraotor Kobe) as a foreign-affiliated pharmaceutical company and is focusing on fostering ecosystems.

Kazuhiro Kanmuri
Inter-Professional Inc.
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Management
Strategy
He engaged in pharmaceutical research and development for more than 25 years. He has gained experience in Japan and in the US development organization of Daiichi Sankyo Co., Ltd., Pfizer Inc., and CRO/consulting organizations, and has been deeply involved in strategic/regulaotry planning of clinical developmentincluding overseas organizations. His specialized fields are extensive, which include company clinical trials and investigator-initiated clinical trials, organization management, strategy development, and clinical trial operation/implementation, safety and pharmacovigillance, and he is active in the academic area of regulatory science. Currently, he engages in the consulting business as an expert in clinical development/organization and human resources development through the corporation that he established by himself and specialized CRO. In addition to being the incumbent, he concurrently served as a visiting lecturer of an academia organization, and he belongs to DIA Japan, an industry association of the pharmaceutical industry, and served as the Vice Program Committee Chair of the DIA Japan annual convention in 2018.

Hidehiro Ando
President & Representative, Andy Pharma Partners Inc.
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Fundraising
- Management
Strategy
He graduated from the Department of Veterinary Medical Science, Faculty of Agriculture, University of Tokyo. He joined Tanabe Pharma Corporation (present Mitsubishi Tanabe Pharma Corporation). After being assigned to the research department of pharmacokinetics, he was involved in partnership-related business of international development department, research and development planning department, etc., including establishment of a joint venture with overseas companies. Subsequently, he worked in the technology transfer department of RIKEN (Institute of Physical and Chemical Research), a regenerative medicine venture, Bayer Yakuhin, a drug discovery venture, and he established a personal office. He has engaged in business development and licensing work for 40 years, and now, based on his experience and networks, he is supporting business development activities, such as licensing agreements and joint research of domestic and foreign pharmaceutical companies/ventures. He provides comprehensive support services ranging from looking for appropriate partners, preparing the draft of contract outline, contract negotiation, and alliance management with partners after the partnership agreement. Especially, based on experience of practicing on both sides of venture and pharmaceutical companies, he keeps in mind providing informative advice and proposals for specific action plans.

Yoshinori Shinoki
Pharma Initiative Support LLC
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Management
Strategy
He graduated from the Department of Biopharmaceutical Sciences, Kyoto Pharmaceutical University, and is a licensed pharmacist. At Sawai Pharmaceutical Co., Ltd., and Eli Lilly Japan K.K., after taking charge of clinical development, business planning, etc., he acquired experience in planning and implementing various clinical development plans as a project leader for newly developed items in the project management department. In addition, he led NHI price negotiation strategy, post-marketing clinical startegy, and market planning. After that, he built up clinical development department at Global CRO. he also led the planning of the clinical development for Japan for small and biological products, as well as negotiating with regulatory authorities. Recently, in addition to medicines, he also led the development of clinical development strategies of medical devices and the products of regenerative medicine (gene therapy products and cell/tissue processed products).

Kenichi Yamahara
Professor at the Laboratory of Molecular and Cellular Therapy, Institute for Advanced Medical Sciences, Hyogo Medical University
Representative Director of CTEX Co., Ltd.
Main specialty areas
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Management
Strategy - Intellectual
property
management
From the time of a former position at the National Cerebral and Cardiovascular Center, he planned and implemented the formulation of the amnion-derived mesenchymal stem cells and investigator-initiated clinical trials for acute grafts versus host disease/Crohn's disease under the support by Health and Labour Sciences Research Grants/AMED. He often had PMDA consultations on the products of regenerative medicine and acquired knowledge of laws and regulations. Taking advantage of the experience, he founded a venture, Japan Biomedical Co., Ltd., which manufactures the domestic bovine serum NeoSERA® for the products of regenerative medicine by satisfying the standards for biological materials in January 2017. Furthermore, in order to aggressively develop investigator-initiated clinical trials of cell therapy making use of university hospital infrastructures, he founded a venture, CTEX Co., Ltd., from Hyogo Medical University in February 2018. Recently, he participated as a medical expert in the company developing the regenerative medical product and is a principal investigator for investigator-initiated clinical trials for the development of cell therapy in the medical device category (adopted by AMED). Currently, he is working on the practice of cell therapy development initiated at the university hospital while concurrently serving in the basic and clinical departments of Hyogo Medical University.

Atsushi Usami
The University of Tokyo Edge Capital Partners Co., Ltd. (UTEC)
Partner and Board Director
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
He holds a Ph.D. in pharmaceutical sciences from the University of Tokyo and is a licensed pharmacist. Prior to joining UTEC in October 2013, he worked as a consultant at Mitsubishi Research Institute. Currently serving as a Partner and Board Director, he oversees venture investments and provides management support primarily in the life sciences sector for seed and early-stage startups. He has played a key role in investments in companies such as OriCiro Genomics, Inc. (acquired by Moderna, Inc.) and Repertoire Genesis, Inc. (acquired by Eurofins Scientific SE). In recognition of his contributions, he was awarded the Encouragement Award for Venture Capitalists at the 23rd Japan Venture Awards in 2023.

Kazushi Kamitani
JPRO INCUBATION OFFICE, FOUNDER
Main specialty areas
- Medicines
- Medical devices
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management - Other
He worked for domestic & international life science firms in the areas of product development, marketing, PR&IR, international business, and business development.
After serving the US subsidiary of a Japanese firm as a board director & CFO and the European startup as CEO, he established the incubation office.
He supports oversea startups to explore Japan market from business & administration side, providing info, advisory and functional services.
International MBA, Certified Specialist of Intellectual Property Management, Financial Planner, IT Coordinator

Daisuke Sugiyama
Hiroshima University, Translational Research Center, Professor
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Fundraising
- Management
Strategy - Intellectual
property
management - Other
He obtained his PhD thesis at University of Tokyo. After working at several hospitals as a clinician, he has engaged in medical reserach at University Paris 6 (French Government Scholar), Dartmouth College (JSPS research fellow), Kyushu University and Hiroshima University. Based on the experiences working at ARO research core hospital and starting up bio-venture company, he supports and conducts both translational and clinical researches.

Junichi Imoto
Chief Venture Capitalist, Nissay Capital Co., Ltd.
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Business
planning - Fundraising
He was involved in research and development of medicinal products at Fujifilm Corporation. Subsequently, he performed duties relating to patent and bibliographic information at Thomson Reuters. Since 2015, he has worked on investment activities mainly in research and development-driven venture companies at Nissay Capital Co., Ltd.

Miyuki Shimizu
Medical Lab Partners Corporation
Main specialty areas
- Medical devices
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Management
Strategy - Intellectual
property
management
She graduated from the Graduate School of Natural Science, Chiba University. She holds the degrees of doctor of medicine and master of management information. She worked on the development of medical devices and pharmaceutical products in a wide range of fields related to transfusions, surgical operations, general-purpose medical devices, dialysis, and rehabilitation at Terumo Corporation. She also has experience in a series of project developments from planning a project to commercializing at a medical practice, as well as experience in management. Since 2015, she has provided consulting services to more than 350 companies entering the medical industry as her current role. She is trying to provide consulting services with the viewpoint of the individual company in mind.

Takato Noumi
Representative Director, FORESIGHT&LINX Cp., Ltd.
Main specialty areas
- Medicines
Specialized support fields
- Business
planning - Fundraising
- Management
Strategy
At his consulting company, he is currently providing practical support for constructing research strategies, business development, and licensing regarding to drug discovery to pharmaceutical companies and biotech venture companies in Japan and overseas. After completing his postgraduate course, he worked in basic research in molecular biology for 10 years followed by joining the Research Division of the headquarters of Novartis. Subsequently he served as the head of theTsukuba Research Laboratories of GlaxoSmithKline K.K. He had engaged in drug discovery research from a global point of view for 15 years. After that, he has been involved in searching for and evaluating drug discovery seeds and technologies and developing international businesses and licensing at Sanofi S.A. in France and his own consulting company for 10 years. He has a broad network of connections with pharmaceutical companies, biotech venture companies, academia, and venture capital firms in Japan and overseas.

Satoshi Fukushima
Globis Capital Partners & Co.
Main specialty areas
- Medical devices
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Fundraising
- Management
Strategy
He graduated from the Faculty of Economics, University of Tokyo. He was involved in investment activities in reformed fields of existing industries, including digital health, at Globis Capital Partners & Co. (GCP). He served as an outside director of several companies, including Medley, Inc., Kakehashi Inc., and Yoriso. Co. Ltd., and provided support to their management personnel mainly in strategy formulation, development of the organization, and finance. In addition to investigation activities, he provided support for fund structuring and open innovations provided by large companies in healthcare fields.
Before joining GCP, he was serving as an M&A advisor mainly in the technology, media, and telecommunication fields and worked on financing at the general headquarters of German Securities Finance Corporation.

Masakatsu Noguchi
SMILE CURVE, Inc.
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Management
Strategy
Ph.D. in life sciences. Worked at consulting firm Dream Incubator, consulting in industry-academia collaboration, business support for tech startups (bio, semiconductor, environmental energy), new business development for large corporations, and overseeing government mega-projects. Previously employed at Abbott Japan and Singapore, managing product marketing for diagnostic tests, pharmaceuticals (women's health), and Asia-Pacific marketing for medical IT. As Business Development Director at Sanamedi, facilitated the commercialization of new medical devices, handled regulatory applications and market launches, and supported investments in and assistance to startups. Additionally served as an evaluator for AMED, and as a mentor for NEDO and university accelerator programs. Currently working independently, he serves as a specially appointed associate professor in the Hiroshima University and as an strategic advisor of MEJ.

Hiroyuki Hasegawa
Director of Life Science, Mitsubishi UFJ Capital Co., Ltd.
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Fundraising
- Management
Strategy
He graduated with a master’s degree from the Faculty of Pharmaceutical Sciences, Hokkaido University. In 1994, he joined Daiichi Seiyaku Company (current Daiichi Sankyo Company, Limited) and was in charge of the areas of infectious diseases and cancers in the Department of Post-marketing Surveillance. In 2004, he joined UFJ Capital Co., Ltd. (current Mitsubishi UFJ Capital Co., Ltd.), where he served as an analyst and capitalist. From 2013, he made an attempt to use open innovation for the Development of Emerging Technologies (OiDE) Fund for the purpose of nurturing outcomes from academia-launched research to become the fundamental technology for drug discovery in collaboration with Daiichi Sankyo Company Limited(liquidated in 2023).Since the first fund in 2017, we have been promoting investment activities using a total of 50 billion yen, including the "Mitsubishi UFJ Life Science Fund No. 4" (20 billion yen).Currently, he holds the position of outside director at Kamuipharma, GAIA Biomedicine, and Luxonus Co. Ltd., as well as serving as a fellow for collaboration between industry and academia at Kyoto University Medical Science and Business Liaison Organization.

Hiroaki Kato
Visiting Professor, The Graduate School of the Digital Hollywood University
Main specialty areas
- Medical devices
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Fundraising
- Management
Strategy
He is a former government official of the Ministry of Health, Labour and Welfare and is working as a doctor specialized in digital health, including telemedicine, AI, and IoT. As an ophthalmologist, he has experience in more than 1,500 surgical operations. He developed instruments for cataract operations and ophthalmic consultation services. He is a rare person who has experience in three areas—medical practice, medical systems, and business—which are required for medical care and the healthcare business, and he understands these areas, providing support for the development of new business covering the full range of medical care. He is also familiar with Japanese medical venture companies and works as a consultant, advisor, and director for large enterprises and venture companies. He wrote 41 books, including Medical Care 4.0 — Medical Care in the Fourth Industrial Revolution Era—.

Tsutomu Uchiyama Ph.,D.
Founder, Uchiyama IP Strategies
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Intellectual
property
management
Patent attorney/Ph.D.
After working as a researcher in molecular biology for Daiichi Pharmaceutical Co., Ltd. (current Daiichi-Sankyo), he passed the examination for patent attorneys in 1996. He worked in the intellectual property departments of three companies as an in-house patent attorney, practitioner, manager and global IP head (Eisai)—Takeda Pharmaceutical Company Limited, SoftBank Investment, and Eisai Co., Ltd.—for a total of 20 years, where he was involved in patent practice and patent strategies, as well as patent portfolio management including patent filing, patent clearance and intellectual property due diligence.
In April 2017, he founded Uchiyama IP Strategies. Currently, utilizing his experience, he mainly assists venture companies, small and medium-sized enterprises, and academia in patent strategy and contractual matters in drug discovery, regenerative medicine, and biotechnology research.

Ayuko Nemoto
Aquaxis Law Office
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Fundraising
- Management
Strategy - Intellectual
property
management
My practice focuses on legal and compliance matters related to healthcare and life science companies. I have been advising on healthcare related laws and regulations, working on legal due diligence for investment, M&A, contract negotiation, and all the other general corporate matters (including domestic and international corporate matters, intellectual properties, labour laws, M&A, JV, IPO support, contract review, personal data, pharmaceutical and advertisement regulations, commercial dispute, accident, communication with relevant authority). Also act as an Advisor of the International Affairs Working Group and MA & Clinical Trial Working Group of Japan Pharmaceutical Industry Legal Affairs Association, Supporter of HealthcareInnovationHub held by Ministry of Economy, Trade and Industry and a member of IRB of the Council for Industrial use of Biological and Environmental Repositories (CIBER). Previously at international law firms.

Takayuki Hirose
Patent attorney, Hirose International Patent Firm
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Intellectual
property
management
He graduated with a master’s degree from the Graduate School, University of Tokyo. Patent attorney. After working in the intellectual property department in a major company, he was involved in lawsuit-, adviser- and application-relating services at a lawyer's office, followed by establishing his own international patent attorney's office in 2017. He offers intellectual property strategies in cooperation with companies as an adviser for multiple companies, including the Japanese Organization for Medical Device Development, Inc., (JOMDD) and Shin Nippon Biomedical Laboratories, Ltd. Furthermore, he applies for patent rights and trademarks in a wide variety of areas. He is involved in intellectual property-related services that include medical devices and pharmaceutical products. When he applies for a patent in foreign countries, he offers application strategies by placing the most importance on client’s profit, for example, to give advice to receive a subsidy or to select a foreign agent at a reasonable price depending on the company’s size.

Katsuya Hashizume
Beyond Next Venutes Inc.
Main specialty areas
- Medical devices
Specialized support fields
- Marketing
- Business
planning - Fundraising
For over 10 years, he has been consistently involved in investing in academia startups. Joined BNV in 2020, focusing on investments in startups in the medical device and digital health sectors. Appointed as an Executive Officer in August 2021. Leads the investment department while also overseeing the community management of portfolio companies. Prior to BNV, he has served as the leader of industry-academic investment group at JAFCO and the chief promoter of JST START. While investing in startups, he has been involved in the company creation of several acedemia startups, including Microwave Chemical (IPO), Biomedical Solutions (M&A) and Bly Medical (M&A). Completed graduate studies at the Keio University Graduate School of Media and Governance.

Kenji Harada
Director & Chief Venture Capitalist, Medical Incubator Japan K.K
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
He graduated with a Ph.D. in Pharmacology from the Faculty of Pharmaceutical Sciences, the University of Tokyo, and is an immunologist by training. He is also a licensed pharmacist and a licensed first-class radiation supervisor. After obtaining his doctorate, he performed a wide range of tasks from basic research to preclinical research as a team leader at Toray Basic Research Laboratories (currently Pharmaceutical Research Laboratories), during which he was developing anti-inflammatory and central nervous system drugs. Subsequently, he was involved in planning and promoting collaborative research with universities and making assessments of platform technologies and drug candidates of American and European biotech startup companies from the technological and intellectual aspects. Furthermore, he conducted GMP inspections in pharmaceutical products and medical device areas multiple times. After starting his career as a venture capitalist, he consistently invested in the biotech sector in the U.S. and Japan. He served as a board member in some of the investees of the U.S. and Japan. Since August 2020, He has expanded his investments into European countries at his current company. He is also engaged in several Japanese governmental projects supporting Japanese startups and research projects.

Kazuhiro Takekita
Osaka University Graduate School of Medicine, Department of Vascular Regeneration and Podiatry, Associate Professor
Main specialty areas
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Intellectual
property
management
He was involved in launching a project of regenerative medicine by using autologous skeletal muscle-derived cell sheets in the Department of Cardiovascular Surgery, Graduate School of Medicine, Osaka University. Subsequently, he was involved in the evaluation of regenerative and biological devices at the Pharmaceuticals and Medical Devices Agency (PMDA). He performed several duties related to the Health Labour Sciences Research Grant, clinical study of human stem cells, and advanced medical care at the Research and Development Division, Health Policy Bureau, Ministry of Health, Labour and Welfare. After that, he led the regenerative medical product evaluation unit as chief of the Office of Review for Regenerative Medical Product, PMDA. Also, he was involved in improving the legislation for regenerative medical products in the revision of the Pharmaceutical Affairs Law in 2014. From August 2017, he has been serving as a specially appointed lecturer in the Department of Cardiovascular Surgery, Graduate School of Medicine, Osaka University, where he promotes projects to develop various products derived from human-induced pluripotent stem cells.

Nobutaka Tani
Chair Person, Nonprofit Organization Japan Organoid Repository
Independent Consultant (New Business in Life Sciences)
Main specialty areas
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Business
planning - Management
Strategy - Intellectual
property
management
He graduated with a master’s degree from the Department of Synthetic Chemistry, Graduate School of Engineering, University of Tokyo. He led the research and development and the launching new project for medical devices, including blood purification system and brain and heart catheters, at Kaneka Corporation. After that, he was involved in RD of the whole life science, including biopharmaceuticals, as the head of the Life Science RD Center, while investing in medical venture companies such as DDS and establishing joint ventures. He then moved to JSR Corporation and established JSR Life Sciences. He led the launch of the life science project for M&A as the first president since JSR Life Sciences was established. As a part of open innovation, he established a CVC and invested in or acquired venture companies in Japan and overseas, while successively holding the positions of president of the invested companies.

Makoto Shigematsu
Representative Director, Kai Fostering Partners Inc.
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
He worked on a new project research and development, trial manufacture, mass production, and commercializing of glass delay line element for VTR at Asahi Glass Co., Ltd. He moved to CSKVC by making use of his relevant experience. As a venture capitalist, he engaged mainly in establishing, financing, fostering, and managing venture companies. He practiced monetizing of novel technologies, timely financing, establishing organization and management, sharing an exit strategy as a milestone, active use of intellectual property rights (open and close), and business talks and negotiations (not bargaining) hated by engineers. He has providing support for company’s growth fostered by competition and cooperation, while being employed by traditional companies for 20 years and by venture companies for 20 years with an understanding exactly the opposite of the corporate culture of both.

Shin-ichi Kamachi
CRO-K Co.,Ltd
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Business
planning - Management
Strategy
Completed doctoral course at Kyushu University Graduate School of Pharmaceutical Sciences. After joining Chugai Pharmaceutical Co., Ltd. Research Institute in 1977, he worked consistently on pharmaceutical research and development. He has experience in approving one small molecule drug and four biopharmaceuticals. He also has the experience of being the first Japanese company to recommend approval for a central review of EMA. He took early retirement in 2004 and mainly assists with biopharmaceutical CMC and non-clinical testing. After establishing the Japanese branch of an American biopharmaceutical consulting company (Biologic Consulting Group, BCG), he currently serves as a senior consultant for CLOCK Co., Ltd., providing support and advice on the development of biopharmaceuticals.

Tamaki Nara
Lecturer, Medical Technology Innovation Center, Juntendo University
Director, Orbis Planning Co., Ltd.
Main specialty areas
- Medical devices
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Management
Strategy
Master of Business Administration(MBA). Field of expertise is innovation management. While working at a major precision equipment manufacturer, engaged in new business, product planning, and corporate planning. Mainly engaged in commercialization in the medical equipment and healthcare field (such as Medical IT), as well as the establishment and management of internal joint ventures. During this time, I became acutely aware of the great significance of building an appropriate collaborative development system between medical researchers and companies, and the necessity of a place for co-creation for both parties to work together. Since then, I have been involved in providing guidance on the implementation of research and development in the medical field and building plans for industry-academia collaboration, leveraging corporate technology and academia's knowledge at medical graduate schools and hospitals. Also have been supporting Start-ups mainly from academia.

Jun Utsumi
CEO, TIR Research Consulting LLC.
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Management
Strategy - Intellectual
property
management
JU graduated from the Graduate School of Veterinary Medicine, Hokkaido University. He holds DVM, D.Sc., MBA and has licenses of Professional Engineer (biotechnology), Radiation Protection Supervisor and Certified Scientist of Medical AI. He was engaged in research and clinical development at Toray Industries, Inc. and succeeded in commercializing the world's first opioid kappa agonist (received Awards from the Pharmaceutical Society of Japan and Okochi Memorial Foundation). After the business career, he served as a professor in Hokkaido University and Kyoto University, as well as an expert in Pharmaceuticals and Medical Devices Agency (PMDA) and a senior consultant for intellectual property at the Japan Agency for Medical Research and Development (AMED). Over 30 years of experience led him to establish a consulting company in 2018, he is also involved in medical DX support and won the Japan Open Innovation Award in 2023. He is part-time lecturers at the graduate schools of the University of Tokyo, Kyoto University, Tokyo Medical and Dental University, and the University of Tsukuba. He published “A Guide to Integrated Strategy in Drug Discovery” (Nanzando Co., 2015).

Yasuo Sasaki
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Management
Strategy - Intellectual
property
management
He graduated with a doctoral degree in science from the Department of Chemistry, Graduate School of Science, Hokkaido University. He was involved in the research and development of pharmaceutical products as an employee of Asahi Kasei Pharma Corporation. He was mainly in charge of preclinical studies and regulatory affairs. From 2013, he provided support for research and development by academia, drug discovery venture companies, and small and medium enterprises centering on the eastern part of Shizuoka Prefecture. Since 2018, he has been providing support services as a freelance advisor or coordinator in the life science field.

 A comprehensive portal site for Medical Innovation Support Office (MEDISO).
A comprehensive portal site for Medical Innovation Support Office (MEDISO).





