Consultationapplication
You can apply to the medical innovation support office (MEDISO) for consultations and support from the comprehensive this portal site.
 What is MEDISO?
What is MEDISO?
MEDISO provides support for business ventures, academia, and individuals intending to put into practical use medicines, medical devices, and regenerative medicinal products.
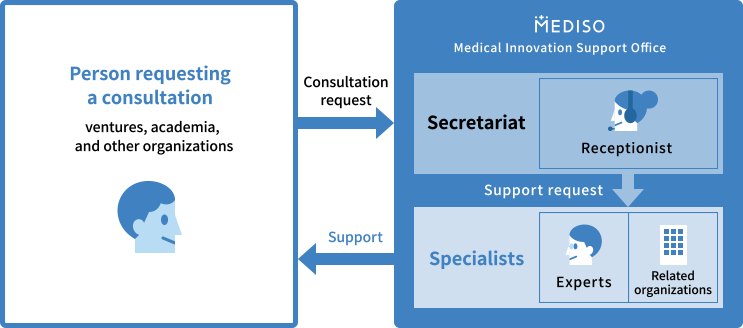
- ●Support targets:Ventures, academic institutions, and individuals aiming to put into practical use the medicines, medical devices, regenerative medicinal products, and novel drug-discovery technologies or medical materials subject to the Pharmaceuticals and Medical Devices Law.
- ●How to apply:Apply using the inquiry form.
- ●Support method:We provide support via phones, e-mail, and meetings.
- ●Support source:Support is provided in cooperation with experts in R&D, consultants in pharmaceutical affairs, and experts from related organizations including the Ministry of Health, Labour and Welfare.
- ●Details of support:MEDISO provides support for the practical application of medicines, medical devices, regenerative medicinal products, and other products subject to the Pharmaceuticals and Medical Devices Law.
That support includes business planning, financing, advice on laws and regulations, and other assistance as required. - ●Remarks:Consultation and support services are free of charge.
Flow of consultation or support
FAQ
- Who can apply for MEDISO consultation?
- Startups, academia, and others seeking to commercialize products subject to the Pharmaceuticals and Medical Devices Act, new drug discovery technologies, medical materials, etc. are eligible to apply.
- Is there a fee for consultation?
- MEDISO consultation is free of charge.
- What kind of support does MEDISO provide?
- MEDISO provides consultation and information regarding commercialization of pharmaceuticals, medical devices, and regenerative medicine products subject to the Pharmaceuticals and Medical Devices Act.
- How does MEDISO provide support?
- Through consultation, experienced MEDISO supporters provide a comprehensive analysis of your issues and advise you on coping strategies and necessary information.
- What is the definition of “Startups and academia”?
- There is not a specific definition. Please contact us for the detail.
- What is the relationship between Pharmaceuticals and Medical Device Agency (PMDA) and MEDISO?
- MEDISO is commissioned by the Ministry of Health, Labour and Welfare (MHLW) and has no direct relationship with PMDA. However, as part of consultation services, MEDISO offers consultation on preparation for PMDA consultation.
- What is the relationship between Healthcare Innovation Hub (InnoHub) and MEDISO?
- InnoHub is a program of the Ministry of Economy, Trade and Industry (METI) that supports companies seeking to commercialize products and health services including products and services outside of the Pharmaceuticals and Medical Devices Act. MEDISO is a program of the Ministry of Health, Labour and Welfare (MHLW) that supports Startups, academia, and others seeking to commercialize products subject to the Pharmaceuticals and Medical Devices Act. InnoHub and MEDISO cooperate with each other to provide appropriate consultation according to the products or services you seek to commercialize and challenges you face.
Mitsubishi Research Institute, Inc.,
Medical Innovation Support Office Secretariat
In case the link does not work, please contact us at the email address below
mediso[at]ml.mri.co.jp (replace [at] to @)
Consultation and support services are free of charge.
 A comprehensive portal site for Medical Innovation Support Office (MEDISO).
A comprehensive portal site for Medical Innovation Support Office (MEDISO).
 Click here for inquiries
Click here for inquiries


