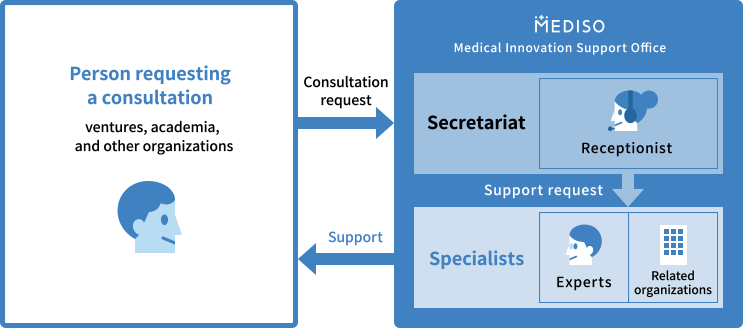
Specialists Introduction
Medical Innovation Support Office support consultants in cooperation with experts (specialists) in each field of R&D, pharmaceutical affairs and insurance, intellectual property management, management and financial accounting, marketing, legal affairs, and international development, as well as related organizations, including the Ministry of Health, Labour and Welfare.
What is Specialists
Experts in each field of R&D, pharmaceutical affairs and insurance, intellectual property management, management and financial accounting, marketing, legal affairs, and international development.
Depending on the specifics of your request, we offer support in cooperation with appropriate specialists and related organizations, including the Ministry of Health, Labour and Welfare.
Introduction of Support Providers
We will introduce registered support providers
(updated as necessary).
Search the area
Search the area
Yutaka Kono
Guest professor
Drug Discovery Science Division,Institute for Open and Transdisciplinary Research Initiatives, Osaka University
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Business
planning - Fundraising
- Management
Strategy - Other

Masaho Ishino
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Management
Strategy - Intellectual
property
management - Other
After engaging in medical research for 25 years, I became a patent attorney (JAPAN). Over the next 20 years, I have supported medical researchers in creating intellectual property and planning research strategies, as well as engaged in contract practice and technology transfer of university IP. Intellectual property strategies for regenerative medicine and other new modalities require not only a high level of expertise in both cutting-edge medical research and intellectual property rights, but also comprehensive knowledge covering clinical development and regulations. Utilizing my experience of contributing to the development research and practical application of university-originated seeds as a representative of an institution responsible for the AMED's Project of Translational and Clinical Research Core Centers and an organizer of nationwide academia intellectual property networks, I aim to contribute to raising the potential of intellectual property of academia/venture.

Katsuhiro Fukunishi
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Other

Takeshi komatani
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Management
Strategy - Intellectual
property
management - Other
Takeshi S Komatani is currently principal litigation certified patent attorney in the field of chemical/pharma and bio/life sciences at a patent law firm, and has a number of counsels, including in academia, start-ups and big pharma internationally. Takeshi received his PhD from the University of Tokyo, Japan, received his LLB and LLM (global legal practice) from Keio University, and is currently a visiting professor at Kobe University, teaching IP strategy in entrepreneurship and Doshisha University (Kyoto). He has completed CEIPI Summer School on IP (University of Strasbourg), France. He is also qualified as a pharmacist with a certificate of Kampo and natural medicines specialist qualification. He was a researcher at F Hoffmann-La Roche in Basle, Switzerland. He has completed the EU-recognised PharmaTrain course at Osaka University, and is qualified as a board-certified member of the Japanese Association of Pharmaceutical Medicine. He is a member of the AIPPI and vice chair of TRIPS SC and a member of IP-GRTK SC and Pharma SC, and a member of the editorial board of the Pharmaceutical Patent Analyst (UK). He is also a member of JPAA, APAA, IPAJ, PSA, JPA, CSAIP and AAAS. He has lectured in academia, including at Japanese and German universities and WIPO Academy, and contributed to a number of articles.

Daisuke Sugiyama
Hiroshima University, Translational Research Center, Professor
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Fundraising
- Management
Strategy - Intellectual
property
management - Other
He obtained his PhD thesis at University of Tokyo. After working at several hospitals as a clinician, he has engaged in medical reserach at University Paris 6 (French Government Scholar), Dartmouth College (JSPS research fellow), Kyushu University and Hiroshima University. Based on the experiences working at ARO research core hospital and starting up bio-venture company, he supports and conducts both translational and clinical researches.

Tetsuomi Takano
t2T Healthcare Inc. CEO/Drug R&D Expert Editor-in-Chief
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Management
Strategy - Other
Tetsuomi Takano possesses over 33 years of clinical development experience spanning multiple therapeutic areas. He has accumulated extensive expertise in development strategy, project management, clinical development, regulatory intelligence, and regulatory affairs across Asian countries and regions including Japan and China. He previously held key positions in the clinical development departments at Astellas Pharma, overseeing processes from IND applications for first-in-human studies to NDA approvals in Asian countries and regions. A graduate of Tokyo University of Science's Faculty of Pharmaceutical Sciences and a RPh, his career began in 1986 at Astellas, where he worked for 31 years till 2017. After working as Senior Strategy Director, Strategy & Planning at Covance (currently Fortrea) from 2017 to 2023, he founded t2T Healthcare Inc. in October 2023. The company is dedicated to providing clients with efficient and distinctive strategic solutions to facilitate operational implementation, clinical development, and IND/NDA programs across the Asia-Pacific region. Additionally, he serves as a member of the PMDA China Expert Committee since 2020 and co-founded the web journal “Drug R&D Expert” in 2021, where he holds the positions of Editor-in-Chief and primary author.

Taku Seriu
Seriu Medical Consulting Ltd. CEO
APCER Life Sciences. Senior Advisor to the Board
The Institute of Drug Development Career Promotion. Representative Director
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Business
planning - Management
Strategy - Other
A physician and Doctor of Medical Science, he began his career as an internist before leading clinical leukemia research at the Universities of Ulm and Heidelberg in Germany. After returning to Japan, he took on leadership roles including Head of Clinical Development at Nihon Schering, Executive Officer at Bristol-Myers Squibb, and Executive Vice President and Board Member at Otsuka Pharmaceutical, where he oversaw drug development, medical affairs, regulatory affairs, pharmacovigilance, and quality assurance.
He has led the development, approval, and post-marketing activities of more than 50 therapeutics and diagnostics across oncology, neuroscience, cardiovascular and renal diseases, infectious diseases, and rare diseases, contributing to global launches and appropriate-use initiatives.
He currently serves as President of Seriu Medical Consulting and Senior Advisor at APCER Life Sciences, supporting R&D strategy, safety and risk management, organizational development, and new business planning. He also advises startups on development strategy, TPP creation, and fundraising pitch preparation, and has a strong track record in post-acquisition organizational integration, aligning cultures, talent, and processes.
He teaches pharmaceutical medicine at several universities and is an editor and contributing author of Introduction to Pharmaceutical Medicine, the first textbook of its kind published in Japan. He continues to practice clinical medicine and remains committed to supporting those driving pharmaceutical innovation in Japan, guided by the principle: “Learn Together, Grow Together.”

Masanori Sato
ReachMed Partners LLC President
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy - Other
Masanori has extensive experience in pharmaceutical R&D, alliance management, and business development. Their career evolved into leadership roles in global product launches, strategic licensing, and early-stage clinical development, particularly in antiviral therapeutics at a R&D focused pharmaceutical division of a leading Japanese conglomerate. Later, as head of the business development department, they managed global licensing agreements and partnerships with major pharmaceutical companies in the U.S. and Europe for multiple in-house pipeline assets. Since 2016, they have held leadership roles in alliance management and business development at leading U.S. biotech and European pharmaceutical companies, focusing on partnerships with Japanese firms, contract negotiations, and search & evaluation of external technologies/assets across Japan and the Asia-Pacific region. From 2021, they joined a top Japanese pharmaceutical company, where they led open innovation initiatives, incubation of internal and external seed assets, and support for venture investments. During this time, they also served as a board member for a portfolio biotech companies. In March 2025, they founded their own company, leveraging their broad expertise and network to support Japanese biotech startups and assist international startups into Japan. They hold national license for Small and Medium Enterprise Management Consultant, among others.

Hitoshi Fujimaki
TMI Associates Attorney-at-law (admitted in Japan and New York)
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Other
Joined TMI Associates in 2016. Completed an LL.M. in National and Global Health Law at Georgetown University Law Center in 2023, earning the Food & Drug Law Certificate. Admitted to the New York State Bar in 2024. The practice focuses on pharmaceutical, medical device, and healthcare regulatory matters in both Japan and the United States, with extensive experience supporting R&D and commercial operations of Japanese companies domestically and abroad. Work includes licensing and collaboration agreements with overseas partners, joint research and development arrangements, CRO agreements, GCP compliance during clinical trials, promotional review, regulatory assessments (including medical-device classification), authority inspections, and broad advisories on pharmaceutical and medical-device regulations. Also advises on U.S. healthcare compliance issues, including the Anti-Kickback Statute, the Sunshine Act, and related federal and state requirements. Author of Introduction to U.S. FDA Drug and Medical Device Law and Regulation (Shojihomu, September 2024).

Satoshi Ogawa
TMI Associates Partner (Attorney)
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Fundraising
- Management
Strategy - Intellectual
property
management - Other
Satoshi Ogawa, Ph.D., is a partner at TMI Associates specializing in life sciences and healthcare. After earning his Ph.D. in Life Sciences from Kyoto University, he joined TMI Associates as an attorney. Following a three-year secondment at a law firm in New Delhi, India, he has been based at TMI’s Kyoto Office since 2019. Dr. Ogawa advises both Japanese and international clients on cutting-edge technologies, including regenerative medicine, digital health, and AI-driven healthcare solutions. His work covers startup support, patent licensing, industry–academia collaboration, regulatory compliance, and intellectual property disputes. He works extensively with universities, startups, pharmaceutical and medical device companies, venture capital firms, and public institutions. He also serves as an advisor to BioCommunity Kansai (BiocK) and HVC KYOTO, and contributes to conflict-of-interest (COI) committees and institutional review boards (IRBs). Dr. Ogawa is passionate about advancing innovation and helping clients navigate complex legal and regulatory challenges in the life science ecosystem. His publications include “Startup Intellectual Property and Legal Guidebook – Key Points for Early-Stage Bio and Life Science Ventures” (METI-Kansai) and “Healthcare Business Legal Consultation Handbook” (Chuokeizai-sha).

Rou Irisawa
IRISAWA Consulting LLC
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Other
Worked at Chugai Pharmaceutical Co., Ltd. for 27 years, primarily in antibody drug research and development, CMC regulatory affairs, and quality assurance, from preclinical trials stage to BLA stage. Involved in the BLA of Japan, US and EU approval for the CTD-Q of Actemra, Japan's original first antibody drug. And also involved in Japan BLA for several biologics Genentech original. Since 2017, involved in the development of many new modalities, including three SAKIGAKE designated cell products, and participated in 21 times PMDA consultations responsible for CMC regulatory and QA for cell products and gene therapy products. In 2023, became independent as a CMC regulatory consultant. Author of ""CMC Pharmaceutical Design - CMC Pharmaceutical Strategy in the Era of New Modality Pharmaceuticals"" (Yakuji Nipposha, 2022), and is the overall supervisor of the Japanese translation of A-CELL (CMC Development Guidance for CAR-T). Fellow at Tama University's Medical Care Solutions Research Institute, and a top performer in the regular course of Pharmaceutical Evaluation Science at the University of Tokyo Graduate School of Pharmaceutical Sciences in 2024. Certified in generative AI skills (November 2025). Currently involved in various projects, including serving as CMC lecturer at seminars on how to proceed with the development of CMC for antibody drugs, cell products, gene therapy products, etc for small company to semi-major company.

Kazufumi Nakamura
Mitsui & Co. Global Investment, Inc. Investment Director
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Business
planning - Fundraising
- Management
Strategy - Other
Kazufumi Nakamura is an Investment Director focused on the investment in biotech in US and China. By leveraging his experiences and networks in Pharma, Biotech and Academia, he connects companies to those experts to expand business and gain scientific insight to help the companies succeed with their important missions.
Kazufumi has over 20 years of healthcare and life sciences experience, spanning business development, investment, entrepreneurship and R&D. Prior to joining MGI, he co-founded a biotech to accelerate innovations through academic institutions in Japan. Previously, he engaged in business development and conducted scientific evaluation, and license-in and license-out of pharmaceutical products at Santen Pharmaceuticals. In addition, he was responsible for creating relationships with the leading academic institutions and innovators, gaining Santen early access to innovation. Prior to Santen, he held a number of clinical development roles at Janssen Pharmaceuticals, including the development of Alzheimer’s disease and Rheumatoid arthritis programs.

keiji Asada
Amenichi Consulting, LLC, CEO
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Fundraising
- Management
Strategy - Other
Graduated from Auburn University with a degree in Chemical Engineering. Currently, serving as the CEO of Amenichi Consulting, LLC, a consulting firm specializing in regulatory compliance (FDA) and marketing support for companies entering the U.S. market, particularly in the fields of pharmaceuticals, medical devices, and regenerative medicine products. With extensive industry experience and expertise, he assists clients in developing business plans, securing funding, and establishing operational structures. Additionally, he provides comprehensive support, from the formulation to the execution of business strategies, to help clients enhance their competitiveness. Furthermore, he leverages his network to match clients with the most suitable business partners. With a global perspective, he offers customized services tailored to the needs of each client, aiming for sustained growth and success. Amenichi was established in 2018 and has supported over 50 companies since its inception.

Yuki Yamakage
Representative Director, Takashi Shimada Office Co., Ltd.
Managing Partner, BD Consulting Ltd.
Main specialty areas
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Other
I have experience working for a global medical device company in insurance strategy development and insurance applications for products in a wide range of medical specialties and disease areas, as well as market access work (relevant academic societies, patient groups, government agencies and industry associations). I have now set up my own private practice and provide support to medical device and pharmaceutical companies and new entrants in terms of healthcare and care systems and insurance strategies. I have experience in the insurance application category (C1/C2 applications) for innovative medical devices. I also have a lot of experience in training employees and conducting seminars, most recently on SaMD insurance applications. My motto is to provide consulting services that address the concerns of my clients after explaining information on insurance applications for medical devices in a way that is easy to understand for beginners.

Tomohisa Hayasaki
GVA LPC Attorney-at-Law / Partner
The Leader of The Team ""Medical / Healthcare / Cosmetic Industry""
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Fundraising
- Intellectual
property
management - Other
After registering as a lawyer, I have been handling disputes such as corporate and labor disputes, as well as providing legal support for the designing lawful business models of new businesses and for funding. In addition, I have been providing legal support for businesses in the field of medical and beauty based on my knowledge of laws relating to medicals including the Japanese Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices and the Japanese Medical Care Act. In particular, I have been providing extensive support for numerous businesses operating in new field such as Software as Medical Device (SaMD) and Non-Software as a Medical Device (Non-SaMD), telemedicine/home call medical services, and also for companies utilizing medical information by employing new technologies such as AI. I am also addressing legal issues faced by companies using advance technologies. Furthermore, I have expertise in the latest marketing trend and offer advice on marketing strategies based on the Japanese Act against Unjustifiable Premiums and Misleading Representations, advertising regulations in Japan such as medical advertising regulations, and medical and pharmaceutical devices regulations.

Tsutomu Hoshiba
Kievit Scientific LLC President
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Other
For over 30 years, I've established and managed Japanese life science companies in the US and Europe. In 2021, I founded Kievit Scientific in Philadelphia, utilizing my Western-acquired experience, up-to-date information, and network. I assist Japanese companies in the pharmaceutical, medical device, and chemical sectors in their international expansion, from strategic planning to product development and market entry. Harnessing my understanding of different cultures and wide-ranging scientific knowledge, I provide solutions to challenges faced by Japanese academia and startups in their global ventures, with the aim of supporting as many Japanese companies as possible to succeed overseas. Furthermore, through these endeavors, I promote the widespread adoption of innovative medical technologies, striving to realize a society where the benefits are extended to many.

Satoshi Morimoto
Morimoto Phrma Partrnering, Representative
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Business
planning - Management
Strategy - Other
Representative of Morimoto Pharma Partnering. Master of Science in Applied Biochemistry, Graduate School of Waseda University. I stared my carrier at the former The Green Cross Corporation (now Mitsubishi Tanabe Pharma Corporation), experienced in pharmaceutical R&D, product strategy planning, and business development. During that time, I was dispatched as the liaison to Germany for four years. My last position was Director of Business Development. Then, I joined to CMIC Holdings, where I led the business development of rare disease drugs as Director of Business Development and Head of IPD Company. From 2016, I spent six years at the Mitsubishi Chemical Group's Life Science Institute(LSII) as head of the Regenerative Medicine Division, where I was involved in the early stages of cell product development, initiating clinical trials, and leading the construction and operation of cell processing facilities. After retiring from LSII in 2021, I started own business as a consultant. I would like to provide practical advice based on my experience in the development and commercialization of rare disease drugs and regenerative medicine products. In addition, I would like to provide practical advice on business development from the perspective of having been introduced to various projects from venture companies over 30 years.

 A comprehensive portal site for Medical Innovation Support Office (MEDISO).
A comprehensive portal site for Medical Innovation Support Office (MEDISO).





