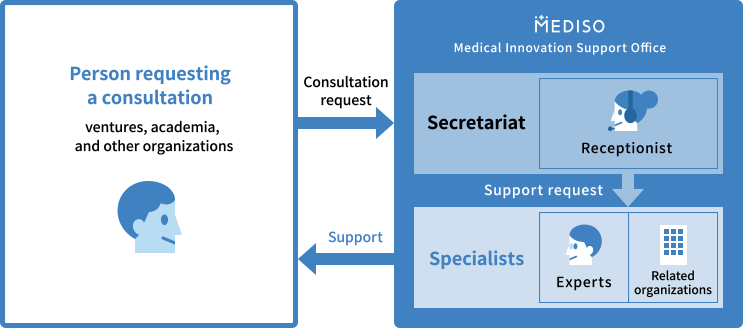
Specialists Introduction
Medical Innovation Support Office support consultants in cooperation with experts (specialists) in each field of R&D, pharmaceutical affairs and insurance, intellectual property management, management and financial accounting, marketing, legal affairs, and international development, as well as related organizations, including the Ministry of Health, Labour and Welfare.
What is Specialists
Experts in each field of R&D, pharmaceutical affairs and insurance, intellectual property management, management and financial accounting, marketing, legal affairs, and international development.
Depending on the specifics of your request, we offer support in cooperation with appropriate specialists and related organizations, including the Ministry of Health, Labour and Welfare.
Introduction of Support Providers
We will introduce registered support providers
(updated as necessary).
Search the area
Search the area
Mirai Miyahara
President of i Access Consulting Inc.
Main specialty areas
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Fundraising
- Management
Strategy
Miyahara holds a Master's degree in Business Administration from the Graduate School of Economics at Tohoku University. She possesses 17 years of professional experience in Regulatory Affairs, QMS, business planning, and overseas marketing at a leading medical device company and Notified bodies in Japan. With a specific focus on multi-country regulatory compliance and extensive experience in overseas medical device markets, Miyahara has demonstrated proficiency in navigating the regulatory landscapes of diverse markets, including Japan, China, India, East Southern Asia, EU and the U.S. She has a proven track record of achieving success in supporting medical companies with regulatory compliance, facilitating international expansion, and establishing strategic partnerships with local entities. Miyahara is also an adviser for JETRO medical devices international expansion program.

Yutaka Kono
Guest professor
Drug Discovery Science Division,Institute for Open and Transdisciplinary Research Initiatives, Osaka University
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Business
planning - Fundraising
- Management
Strategy - Other

Akihiko Watanabe, Ph.D.
President, GlobalHumanScienceInnovation; Senior Consultant, Headland Strategy Group; Visiting Professor, Gifu Pharmaceutical University; BD Consultant, United Immunity; Executive Advisor, SOKK Japan
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy
With R&D expertise atJapanese and global pharmaceutical companies as a backbone, he has been engaged in marketing, BD, management planning and fundraising, in addition to R&D. In the Editorial Committee of the Pharmaceutical Society of Japan, he organized roundtable discussions and special issues on practical application of academia seeds. He has been thinking about how Japanese bio-ventures can succeed. Over the past five years, he has worked at bio-venture companies as CBO or VP of BD and open innovation. He is also engaged in the making and analysing stratetgies of management, business, R&D, and marketing for pharmaceutical companies and bio-ventures on commission from a US consulting firm.

Eiichi Yamaguchi
Eiichi Yamaguchi, Ph.D. President A1 Partners
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy
Ph.D from Faculty of Pharmaceutical Sciences Kyushu University. Currently engaged in management and business development consulting for pharmaceutical companies, biotech ventures, and consulting firms. Over 20 years expertise of business development at Shionogi Co., Ltd., a major pharmaceutical company as well as bioventure companies. Senior Director, Business Development & Corporate Planning Dept., Shionogi. A lot of global business experience such as the first Managing Director, Shionogi Singapore, Vice President, Shionogi Qualicaps, Inc., North Carolina, and Shionogi New York. Board of Director & President & COO, a bioventure company. The first chairman, Japan Pharma Alliance Association. The Vice Chairman, Public Private Alliance Conference associated with Infectious diseases led by Dr. Shigeru Omi. Also engaged in Director, International Committee, JPMA. Last but not least, selected as Mentor for Blockbuster Tokyo 2021

Masakazu Masujima
Partner, Mori Hamada & Matsumoto
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Fundraising
- Management
Strategy - Intellectual
property
management
Masa is a guru of startup legal advisory space in Japan. Based on the experiences at Palo Alto office of Wilson Sonsini Goodrich & Rosati, his advice is West Coast style; progressive, risk-base and proactive. Through more than 2 dacades of dedication to the startup space, he has advised IPOs, M&As and major cross-border fundraising for may startups, including biotech and healthcare ones, and have also worked with entrepreneurs through a variety of touch situation, such as disputes between founders, problems with investors as well as intellectual property disputes. Masa has served as a council member of numerous Government committees, such as Regulatory Reform Promotion Council, the Regulatory Sandbox Committee, the Digital Market Competition Council and the Industrial Structure Council. He is admitted to NY bar and Japan bar, and a licensed patent attorny in Japan.

Yoshiki Kawabata
President, MedX Inc.
Main specialty areas
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
Advantage from over 35 years of multiple company & responsibility experience in medical device industry, helping foreign companies to enter into Japanese market. Key highlights in the past achievements are : with Johnson & Johnson Mdecial Company, worked with US R&D team to develop new product which reflecting Japanese patient and physicians needs - three years resided in Cincinnati, Ohio: HeartFlow Inc., which developed the first "program device" - FFRCT, enabling non-invasive detection of FFR, applied and received regulatory approval and reimbursement as a first product and company. : in Palette Lifesciences Japan, establishment of new subsidiary for start-up company which transferred the business from exclusive distributor. Started with minimum number of direct resources and having multiple partnerships with logistics, finance and regulatory affairs, revitalized the business and achieved a 149% growth over the last year of distributor.

Nao Yoshizawa
GRiT Partners Law offices/Willsame.co.ltd/Cabinet office advisor for National bioecnomy strategy/Keio university Medical school
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
After leaving the law firm Nishimura Asahi, [the individual] established the law firm GRiT Partners and assumed the role of managing partner. They also serve as the representative of Willsame Corporation and hold qualifications as a lawyer, patent attorney, CFE (Certified Fraud Examiner), Applied Information Technology Engineer, Information Processing Security Support Engineer, and IT Strategist. They have served as a committee member for the formulation of standards in electronic commerce and related information transactions at the Ministry of Economy, Trade and Industry, as an expert in the Cabinet Office's bioecnomy strategy, and as an advisor to Biock. They have been involved in the establishment of the Japan Chapter of Aging2.0 in Silicon Valley and served as a mentor for Alchemist Accelerator. A graduate of the law faculty at Hitotsubashi University, they completed intellectual property and machine learning programs at Stanford University, as well as multiple AI programs at MIT. They serve as a program committee member for the Graduate School of Pharmaceutical Sciences at the University of Tokyo and the Medical Innovation Human Resources Development Course at Tokyo Medical and Dental University. With a deep understanding of international perspectives on digital health, they provide advisory support for startups and pharmaceutical companies' DX (Digital Transformation) programs. They founded the Life Science Incubation Council and focus on launching projects and connecting them with ecosystems both domestically and internationally.

Kiyoaki Kojima
Partner
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
Kiyo advises clients in corporate formation, governance, and compliance matters; mergers and acquisitions; joint ventures; leasing and licensing matters; distribution and franchise arrangements; and a wide range of commercial transactions.An important part of Kiyo’s practice includes his work with international companies in relocating and/or establishing ventures in the U.S. He assists companies and investors with incentives and site selection; real estate acquisitions and construction agreements; corporate formation and governance; asset purchases and joint ventures; regulatory compliance; and contracts of all types to help ensure a seamless U.S. launch or transition.He also counsels clients in virtually all areas of labor and employment law, including drafting and/or interpretation of employment agreements, company policies, and handbooks; union-avoidance training; countering discrimination and harassment claims; hire, fire and discipline issues; and dispute resolution procedures. Active in the community, Kiyo is a member of the Atlanta Bar Association, a board member of the Japan America Society of Georgia, and a board member of the Japanese Chamber of Commerce of Georgia. He also serves in “advisor” or “supporter” roles in various entities in Japan, such as InnoHub, MEDISO, and IDEC Yokohama.

Tomokatsu Hongo
Keio Innovation Initiative, Inc
Corporate Officer
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Business
planning - Fundraising
- Management
Strategy
Ph.D. in Biological Chemistry from Nagoya University. At Summit Pharmaceuticals International Corporation, I was engaged in VC fund management, investment and sales support for bio-ventures, and domestic sales agency business for overseas bio-ventures. In 2016, I joined Keio Innovation Initiative (KII) as an investment manager in the medical and health field. I have been investing in drug discovery, regenerative medicine, and medical device ventures by leveraging his business experience in the pharmaceutical industry and career as a researcher, and providing a wide range of hands-on support, not only in terms of funding, but also in business development.

Takahiro Mizumoto
Ant innovations Co., Ltd
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
Kyoto University Graduate School of Medicine, Master’s Program in Intellectual Property Management.
After passing the Japanese Patent Attorney Examination, gained venture investment experience in high-tech sectors including IT and healthcare at NIFS SMBC Capital (later split into Daiwa Corporate Investment and SMBC Venture Capital).
Subsequently joined Showa Shell Sekiyu, where he led the planning and development of multiple new services spanning payment solutions, retail electricity, and mobile app businesses.
With the launch of the University of Tokyo Innovation Platform (UTokyo IPC), he joined the firm and founded 1stRound, a cross-university incubation program. He later spearheaded the establishment of an approximately JPY 25 billion open-innovation promotion fund and served as its Chief Investment Officer.
In November 2025, he founded Ant Innovations, a newly established venture capital firm and subsidiary of the private equity firm Ant Capital Partners, and assumed the role of Representative Director and Partner.

Chia-Feng Lu
Shareholder(Senior Partner), Greenberg Traurig, LLP
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
Chia-Feng Lu represents life sciences companies and industry associations in strategic regulatory and legislative worldwide to guide and develop new legislation and policy, to frame effective approaches to working with the various agencies on compliance and investigation issues. He has intensive experience in provision of the guidance to companies introducing novel technology products, such as AI, block chain, 3D-printing, cancer immunotherapy, digital health, cell / gene therapy, precision medicine-related products and services, RNAi drug, as well as SMART device. In his past experience, he has already assisted companies in translating the scientific ideas from Nobel Laureates into the successful commercial launch of at least two products. In addition, he advises investment banks, private equity firms, and venture capital groups on their evaluations of the technologies, deal structures, and the resulting business impacts, as well as regulatory uncertainty with respect to novel technology and compliance. He specializes in various issues in connection with biotech investors or life sciences companies’ research & development strategy, portfolio management, corporate strategy planning, partnerships, licensing, project finance, auction, investment, and M&A. He has also served as an adjunct faculty member of a number of leading academic institutions in the U.S. and Japan, and an advisor to numerous government agencies.

Hitoshi Takuma
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Fundraising
- Management
Strategy - Intellectual
property
management

Masashi Matsunaga
Biospire Japan Ltd. / Biospire UK Ltd.
CEO
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy
As an experienced global business development strategist, Dr Masashi Matsunaga provides business soft-landing, clinical and regulatory support to the UK and European market. He also enables to support IP management, marketing and pitch-deck brush-up in the MEDISO support package. He is based at Harwell Science & Innovation Campus, a core UK innovation hub located near Oxford.

Shunichi Takahashi
Bayer Yakuhin, Ltd.
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy
After joining Mitsui Pharmaceuticals Co., Ltd., he worked at Nihon Schering K.K., Berlex Biosciences (a US subsidiary of Schering AG). In 2007, he joined Bayer due to management integration. Over the past 15 years, he was in charge of exploring new projects and verifying concepts in drug discovery research, especially in the areas of cardiovascular systems, immunity, stem cell research, etc. Subsequently, he was in charge of the project management cardiovascular system area manager of development headquarters, the primary care manager of the medical affairs headquarters, and he has been in his current role since 2014 (Open Innovation Center director). He leads strategic partnerships and joint research with Japanese academies and venture companies. He is also verifying new pharmaceutical business models that take advantage of big data, AI, IoT, and others. In 2018, he launched the first venture incubation facility (CoLaboraotor Kobe) as a foreign-affiliated pharmaceutical company and is focusing on fostering ecosystems.

Hidehiro Ando
President & Representative, Andy Pharma Partners Inc.
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Fundraising
- Management
Strategy
He graduated from the Department of Veterinary Medical Science, Faculty of Agriculture, University of Tokyo. He joined Tanabe Pharma Corporation (present Mitsubishi Tanabe Pharma Corporation). After being assigned to the research department of pharmacokinetics, he was involved in partnership-related business of international development department, research and development planning department, etc., including establishment of a joint venture with overseas companies. Subsequently, he worked in the technology transfer department of RIKEN (Institute of Physical and Chemical Research), a regenerative medicine venture, Bayer Yakuhin, a drug discovery venture, and he established a personal office. He has engaged in business development and licensing work for 40 years, and now, based on his experience and networks, he is supporting business development activities, such as licensing agreements and joint research of domestic and foreign pharmaceutical companies/ventures. He provides comprehensive support services ranging from looking for appropriate partners, preparing the draft of contract outline, contract negotiation, and alliance management with partners after the partnership agreement. Especially, based on experience of practicing on both sides of venture and pharmaceutical companies, he keeps in mind providing informative advice and proposals for specific action plans.

Atsushi Usami
The University of Tokyo Edge Capital Partners Co., Ltd. (UTEC)
Partner and Board Director
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
He holds a Ph.D. in pharmaceutical sciences from the University of Tokyo and is a licensed pharmacist. Prior to joining UTEC in October 2013, he worked as a consultant at Mitsubishi Research Institute. Currently serving as a Partner and Board Director, he oversees venture investments and provides management support primarily in the life sciences sector for seed and early-stage startups. He has played a key role in investments in companies such as OriCiro Genomics, Inc. (acquired by Moderna, Inc.) and Repertoire Genesis, Inc. (acquired by Eurofins Scientific SE). In recognition of his contributions, he was awarded the Encouragement Award for Venture Capitalists at the 23rd Japan Venture Awards in 2023.

Daisuke Sugiyama
Hiroshima University, Translational Research Center, Professor
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Fundraising
- Management
Strategy - Intellectual
property
management - Other
He obtained his PhD thesis at University of Tokyo. After working at several hospitals as a clinician, he has engaged in medical reserach at University Paris 6 (French Government Scholar), Dartmouth College (JSPS research fellow), Kyushu University and Hiroshima University. Based on the experiences working at ARO research core hospital and starting up bio-venture company, he supports and conducts both translational and clinical researches.

Junichi Imoto
Chief Venture Capitalist, Nissay Capital Co., Ltd.
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Business
planning - Fundraising
He was involved in research and development of medicinal products at Fujifilm Corporation. Subsequently, he performed duties relating to patent and bibliographic information at Thomson Reuters. Since 2015, he has worked on investment activities mainly in research and development-driven venture companies at Nissay Capital Co., Ltd.

Ayuko Nemoto
Aquaxis Law Office
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Fundraising
- Management
Strategy - Intellectual
property
management
My practice focuses on legal and compliance matters related to healthcare and life science companies. I have been advising on healthcare related laws and regulations, working on legal due diligence for investment, M&A, contract negotiation, and all the other general corporate matters (including domestic and international corporate matters, intellectual properties, labour laws, M&A, JV, IPO support, contract review, personal data, pharmaceutical and advertisement regulations, commercial dispute, accident, communication with relevant authority). Also act as an Advisor of the International Affairs Working Group and MA & Clinical Trial Working Group of Japan Pharmaceutical Industry Legal Affairs Association, Supporter of HealthcareInnovationHub held by Ministry of Economy, Trade and Industry and a member of IRB of the Council for Industrial use of Biological and Environmental Repositories (CIBER). Previously at international law firms.

Kenji Harada
Director & Chief Venture Capitalist, Medical Incubator Japan K.K
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
He graduated with a Ph.D. in Pharmacology from the Faculty of Pharmaceutical Sciences, the University of Tokyo, and is an immunologist by training. He is also a licensed pharmacist and a licensed first-class radiation supervisor. After obtaining his doctorate, he performed a wide range of tasks from basic research to preclinical research as a team leader at Toray Basic Research Laboratories (currently Pharmaceutical Research Laboratories), during which he was developing anti-inflammatory and central nervous system drugs. Subsequently, he was involved in planning and promoting collaborative research with universities and making assessments of platform technologies and drug candidates of American and European biotech startup companies from the technological and intellectual aspects. Furthermore, he conducted GMP inspections in pharmaceutical products and medical device areas multiple times. After starting his career as a venture capitalist, he consistently invested in the biotech sector in the U.S. and Japan. He served as a board member in some of the investees of the U.S. and Japan. Since August 2020, He has expanded his investments into European countries at his current company. He is also engaged in several Japanese governmental projects supporting Japanese startups and research projects.

Makoto Shigematsu
Representative Director, Kai Fostering Partners Inc.
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
He worked on a new project research and development, trial manufacture, mass production, and commercializing of glass delay line element for VTR at Asahi Glass Co., Ltd. He moved to CSKVC by making use of his relevant experience. As a venture capitalist, he engaged mainly in establishing, financing, fostering, and managing venture companies. He practiced monetizing of novel technologies, timely financing, establishing organization and management, sharing an exit strategy as a milestone, active use of intellectual property rights (open and close), and business talks and negotiations (not bargaining) hated by engineers. He has providing support for company’s growth fostered by competition and cooperation, while being employed by traditional companies for 20 years and by venture companies for 20 years with an understanding exactly the opposite of the corporate culture of both.

Masanori Sato
ReachMed Partners LLC President
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy - Other
Masanori has extensive experience in pharmaceutical R&D, alliance management, and business development. Their career evolved into leadership roles in global product launches, strategic licensing, and early-stage clinical development, particularly in antiviral therapeutics at a R&D focused pharmaceutical division of a leading Japanese conglomerate. Later, as head of the business development department, they managed global licensing agreements and partnerships with major pharmaceutical companies in the U.S. and Europe for multiple in-house pipeline assets. Since 2016, they have held leadership roles in alliance management and business development at leading U.S. biotech and European pharmaceutical companies, focusing on partnerships with Japanese firms, contract negotiations, and search & evaluation of external technologies/assets across Japan and the Asia-Pacific region. From 2021, they joined a top Japanese pharmaceutical company, where they led open innovation initiatives, incubation of internal and external seed assets, and support for venture investments. During this time, they also served as a board member for a portfolio biotech companies. In March 2025, they founded their own company, leveraging their broad expertise and network to support Japanese biotech startups and assist international startups into Japan. They hold national license for Small and Medium Enterprise Management Consultant, among others.

Satoshi Ogawa
TMI Associates Partner (Attorney)
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Fundraising
- Management
Strategy - Intellectual
property
management - Other
Satoshi Ogawa, Ph.D., is a partner at TMI Associates specializing in life sciences and healthcare. After earning his Ph.D. in Life Sciences from Kyoto University, he joined TMI Associates as an attorney. Following a three-year secondment at a law firm in New Delhi, India, he has been based at TMI’s Kyoto Office since 2019. Dr. Ogawa advises both Japanese and international clients on cutting-edge technologies, including regenerative medicine, digital health, and AI-driven healthcare solutions. His work covers startup support, patent licensing, industry–academia collaboration, regulatory compliance, and intellectual property disputes. He works extensively with universities, startups, pharmaceutical and medical device companies, venture capital firms, and public institutions. He also serves as an advisor to BioCommunity Kansai (BiocK) and HVC KYOTO, and contributes to conflict-of-interest (COI) committees and institutional review boards (IRBs). Dr. Ogawa is passionate about advancing innovation and helping clients navigate complex legal and regulatory challenges in the life science ecosystem. His publications include “Startup Intellectual Property and Legal Guidebook – Key Points for Early-Stage Bio and Life Science Ventures” (METI-Kansai) and “Healthcare Business Legal Consultation Handbook” (Chuokeizai-sha).

Saho Murakami
VISIONEO LLC CEO
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Business
planning - Fundraising
- Management
Strategy
After gaining clinical experience as an ophthalmologist, I served at the Pharmaceuticals and Medical Devices Agency (PMDA), where I was responsible for reviewing and providing consultations on pharmaceuticals, medical devices, and regenerative medical products in the fields of CNS, sensory organs, and anesthesiology. My work included designation reviews for Sakigake (pioneering) and orphan drugs, as well as post-marketing safety activities. Currently, I support business and clinical development across the healthcare and life sciences sectors, offering strategic and R&D advisory services to major corporations, startups, and venture capital firms.

Aiko Kato Sullenberger
RykoTECH President
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Marketing
- Business
planning - Fundraising
- Management
Strategy
Aiko Kato Sullenberger, CEO of RykoTECH, a significant contributor within the Boston startupecosystem for several years, brings a wealth of experience in various roles, spanning investor,startup, and accelerator. She provides specialized advisory services comprehensive strategicconsultation and facilitation of market entry into the US across diverse sectors, including drugdiscovery, medical devices, digital health, digital therapeutics and biotech. Aiko can help • Fundraising • Strategic Partnership Development • Create Deal Flow (Connecting with Potential Investors) • Identifying Partners and Advisors • Assistance in Facilitating Clinical Trials • Corporate Strategy: Investment Thesis, Portfolio Analysis • Market / Trend Research & Analysis • Go-to-Market Strategy • Academic Business Liaison: Interdisciplinary Project Lead • Cross-Functional Team Building

Kazufumi Nakamura
Mitsui & Co. Global Investment, Inc. Investment Director
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Business
planning - Fundraising
- Management
Strategy - Other
Kazufumi Nakamura is an Investment Director focused on the investment in biotech in US and China. By leveraging his experiences and networks in Pharma, Biotech and Academia, he connects companies to those experts to expand business and gain scientific insight to help the companies succeed with their important missions.
Kazufumi has over 20 years of healthcare and life sciences experience, spanning business development, investment, entrepreneurship and R&D. Prior to joining MGI, he co-founded a biotech to accelerate innovations through academic institutions in Japan. Previously, he engaged in business development and conducted scientific evaluation, and license-in and license-out of pharmaceutical products at Santen Pharmaceuticals. In addition, he was responsible for creating relationships with the leading academic institutions and innovators, gaining Santen early access to innovation. Prior to Santen, he held a number of clinical development roles at Janssen Pharmaceuticals, including the development of Alzheimer’s disease and Rheumatoid arthritis programs.

keiji Asada
Amenichi Consulting, LLC, CEO
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Fundraising
- Management
Strategy - Other
Graduated from Auburn University with a degree in Chemical Engineering. Currently, serving as the CEO of Amenichi Consulting, LLC, a consulting firm specializing in regulatory compliance (FDA) and marketing support for companies entering the U.S. market, particularly in the fields of pharmaceuticals, medical devices, and regenerative medicine products. With extensive industry experience and expertise, he assists clients in developing business plans, securing funding, and establishing operational structures. Additionally, he provides comprehensive support, from the formulation to the execution of business strategies, to help clients enhance their competitiveness. Furthermore, he leverages his network to match clients with the most suitable business partners. With a global perspective, he offers customized services tailored to the needs of each client, aiming for sustained growth and success. Amenichi was established in 2018 and has supported over 50 companies since its inception.

Hikaru Saito
Saisei Ventures LLC Partner
Main specialty areas
- Medicines
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
As a partner of Saisei Ventures, a newly established global venture capital firm that invests in advanced biotech companies in cell and gene therapy, Dr. Saito is responsible for Japan operations including company creation, integrating Japanese science and technology with the Western ecosystem. Prior to joining Saisei, he started career as a research scientist at Astellas Pharma Inc. and then as a Senior Manager of Business Development and as a Senior Investment Manager at Astellas Venture Management, a CVC arm based in Silicon Valley, USA, where he was a lead role for deal process, due diligence and transactions for venture investments in RNA therapeutics, cell and gene therapy. He was providing business support to portfolio companies based on the expertise, experience, and global network. He also led the development of strategic partnerships with venture capital funds, accelerators, and institutional investors based in the U.S. and Europe. Prior to joining Astellas, he was a Visiting Fellow at the Institute of Medical Science, University of Tokyo, and a Research Fellow of the Japan Society for the Promotion of Science. He received his B.S. in Biotechnology and M.S. and Ph.D. in Biomolecular Molecularl Engineering from Tokyo Institute of Technology.

Akiyo Inoko Hewett
Attorney, Smith, Gambrell & Russell, LLP
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
Akiyo Inoko Hewett is a bilingual and double-licensed attorney based in Atlanta, Georgia, United States. She graduated from Tokyo University of Foreign Studies and earned her J.D. at Keio Law School. After passing the Japanese bar exam and completing her legal training in Kushiro, Hokkaido, Akiyo relocated to the United States. She earned an LL.M. from Emory University School of Law in Atlanta, Georgia. Currently, Akiyo serves as an attorney at Smith, Gambrell & Russell, LLP, where she provides legal services to Japanese companies doing business in the United States and supports clients across a broad range of corporate matters. Her practice areas include international and domestic commercial transactions, regulartory compliance, healthcare law, and immigration law.

Tomohisa Hayasaki
GVA LPC Attorney-at-Law / Partner
The Leader of The Team ""Medical / Healthcare / Cosmetic Industry""
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Fundraising
- Intellectual
property
management - Other
After registering as a lawyer, I have been handling disputes such as corporate and labor disputes, as well as providing legal support for the designing lawful business models of new businesses and for funding. In addition, I have been providing legal support for businesses in the field of medical and beauty based on my knowledge of laws relating to medicals including the Japanese Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices and the Japanese Medical Care Act. In particular, I have been providing extensive support for numerous businesses operating in new field such as Software as Medical Device (SaMD) and Non-Software as a Medical Device (Non-SaMD), telemedicine/home call medical services, and also for companies utilizing medical information by employing new technologies such as AI. I am also addressing legal issues faced by companies using advance technologies. Furthermore, I have expertise in the latest marketing trend and offer advice on marketing strategies based on the Japanese Act against Unjustifiable Premiums and Misleading Representations, advertising regulations in Japan such as medical advertising regulations, and medical and pharmaceutical devices regulations.

Kunishige Masui
Managing Partner
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Fundraising
- Intellectual
property
management
Graduated from Kyoto University Faculty of Law, University of Tokyo Law School, and University of California, Irvine LLM. 2014 - 2021 at Nagashima Ohno & Tsunematsu; 2020 - 2021 at Smith, Gambrell & Russell, LLP (US law firm). Established Masui & Partners in December 2021. Mr. Kunishige Masui assisted numerous startups, from angel rounds to companies nearing IPO, and has also assisted in the launch of many new businesses, including cross-border localization. He is well versed in the legal and financing issues that arise for startups and new business development, and provides support as a mentor for JETRO, Healthcare Innovation Hub, Kawasaki-NEDO Innovation Center (K-NIC), Venture Café Tokyo, and IDEC. In addition to providing easy-to-understand explanations of foreign legal systems in comparison to those in Japan, he also specializes in removing obstacles to business growth by not only providing answers to "points that clients have actually consulted with" but also by digging into "points that need attention that clients are not yet aware of.”

Kensuke Suzuki
Nagashima Ohno & Tsunematsu
Partner, Attorney-at-law
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Business
planning - Fundraising
- Management
Strategy - Intellectual
property
management
Kensuke Suzuki is a partner at Nagashima Ohno & Tsunematsu. He represents both domestic and international clients, with a special focus on cross-border transactions, with respect to pharmaceutical, medical and healthcare business, M&A and corporate transactions, fund formation and management, and financial regulations. With expert knowledge of the Japanese medical, pharmaceutical, medical device, and other various healthcare-related regulations, he advises on a wide variety of business activities in the broad healthcare sector, such as regulatory compliance, data protection, mergers and acquisitions, alliances, business startups, financing, licensing and other commercial transactions. He was admitted to the Japan Bar in 2000 and worked as visiting attorney at Kirkland & Ellis (Chicago) from 2006 to 2007. He served as a special adviser to the Ministry of Health, Labour and Welfare from 2014 to 2015. He is fluent in Japanese and English.

Hiroyuki Hasegawa
Director of Life Science, Mitsubishi UFJ Capital Co., Ltd.
Main specialty areas
- Medicines
- Medical devices
- Regenerative medicinal products
Specialized support fields
- Laws and
regulations - Marketing
- Business
planning - Fundraising
- Management
Strategy
He graduated with a master’s degree from the Faculty of Pharmaceutical Sciences, Hokkaido University. In 1994, he joined Daiichi Seiyaku Company (current Daiichi Sankyo Company, Limited) and was in charge of the areas of infectious diseases and cancers in the Department of Post-marketing Surveillance. In 2004, he joined UFJ Capital Co., Ltd. (current Mitsubishi UFJ Capital Co., Ltd.), where he served as an analyst and capitalist. From 2013, he made an attempt to use open innovation for the Development of Emerging Technologies (OiDE) Fund for the purpose of nurturing outcomes from academia-launched research to become the fundamental technology for drug discovery in collaboration with Daiichi Sankyo Company Limited(liquidated in 2023).Since the first fund in 2017, we have been promoting investment activities using a total of 50 billion yen, including the "Mitsubishi UFJ Life Science Fund No. 4" (20 billion yen).Currently, he holds the position of outside director at Kamuipharma, GAIA Biomedicine, and Luxonus Co. Ltd., as well as serving as a fellow for collaboration between industry and academia at Kyoto University Medical Science and Business Liaison Organization.

 A comprehensive portal site for Medical Innovation Support Office (MEDISO).
A comprehensive portal site for Medical Innovation Support Office (MEDISO).





